Drug-like chelating agents: a potential lead for Alzheimer's disease
Abstract
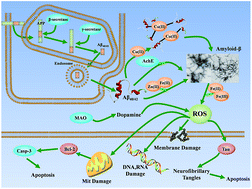
Recent evidence has indicated that dyshomeostasis of biometals (Fe, Cu, Zn) in the brain might contributes to the pathogenesis of Alzheimer's disease (AD). Experiments have also found that the level of metal ions in AD patients was 3–7 fold higher than that of healthy individuals. Many drug-like chelating agents were found to be able to reverse Aβ aggregation, dissolve amyloid plaques and delay the AD-related cognitive impairment. This review systematically discusses the drug-like chelating agents for AD as well as their potential applications in the future.
- This article is part of the themed collection: Towards understanding and treating Alzheimer’s disease

 Please wait while we load your content...
Please wait while we load your content...