Tween20 surfactant effect on the electrocatalytic performance of porous carbon micro-spheres supported MnO2
Abstract
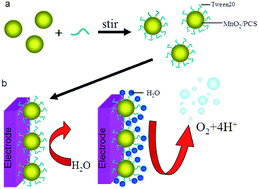
This study is concerned with the electrocatalytic evolution of oxygen at polyoxyethylene sorbitan monolaurate (Tween20) modified porous carbon micro-spheres (PCS) supported MnO2 catalyst in 0.1 M KOH solution. The Super P (SP) was used to improve the electrical conductivity of the catalyst. The electrochemical measurements revealed a significant enhancement of the electrocatalytic activity of the MnO2/PCS towards the oxygen evolution reaction (OER) upon the Tween20 modification. The onset potentials of the OER at the Tween20 modified MnO2/PCS particles electrode are more negative by about 0.162 V compared with the bare (i.e. unmodified) MnO2/PCS electrode. Tween20 is absorbed on the surface of MnO2/PCS and covers the entire surface of the MnO2/PCS particles homogeneously. The Tween20 plays a vital role as a catalytic mediator, which facilitates the ion transfer during the water oxidation into molecular oxygen and thus the OER is accomplished at less positive potentials.

 Please wait while we load your content...
Please wait while we load your content...