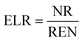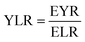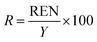Assessing the critical role of ecological goods and services in microalgal biofuel life cycles†
George G. Zaimes and
Vikas Khanna*
Department of Civil and Environmental Engineering, University of Pittsburgh, Pittsburgh, Pennsylvania 15261, USA. E-mail: khannav@pitt.edu
First published on 5th September 2014
Abstract
Microalgal bioenergy systems are gaining attention as a commercial biotechnical platform for producing renewable transportation fuels. In recent years, process-based life cycle assessment (LCA) has been extensively applied to understand the life cycle environmental impacts of emerging microalgal biofuel systems. However, conventional process-based LCA fails to account for the role of ecological goods and services within fuel and product life cycles. Additionally, traditional life cycle energy analysis suffers from several limitations such as ignoring the difference in quality and substitutability of resources, and accounting for only the first law of thermodynamics. To address these shortcomings, a hybrid Ecologically based-LCA (EcoLCA) model is developed to quantify the contribution of ecological resources within the algae-to-fuel supply chain and to compare the resource intensity of producing microalgal derived renewable diesel (RD) to that of petroleum diesel (PD). Multiple thermodynamic return on investment (ROI) metrics and performance indicators are used to quantify the consumption of ecological goods and services, environmental impacts, and resource intensity of producing microalgal RD. Results indicate that the quality corrected thermodynamic return on investment and renewability index for microalgal RD ranges from 0.17 to 0.44 and 3.51% to 6.36% respectively, depending on the choice of coproduct options and processing technologies. This work reveals that algae-to-fuel systems are highly dependent on non-renewable ecological resources reflected in their low renewability index; have a low quality corrected thermodynamic ROI (<1) and thus are not energetically viable; and are more ecologically resource intensive as compared to their petroleum equivalent—potentially negating their environmental benefits.
1. Introduction
Emerging issues of global climate change, domestic energy security concerns, and regulatory renewable fuel mandates are driving the production of low carbon biofuels.1 However, there is concern that the production of first generation biofuels—fuels derived from arable crops such as corn or soybean may displace or compete with cropland, potentially reducing the quantity of food crops available for human/livestock consumption. This could have major economic consequences including food shortages and inflation of global food prices.2,3 Previous analysis has also shown that first generation biofuels have marginal energy returns2 and provide limited greenhouse gas (GHG) emissions reductions relative to petroleum fuels.4–7 Additionally, the production of first generation biofuels may result in increased ecosystems degradation including impacts on biodiversity, water, soil and forest resources.8,9 Recently, liquid transportation fuels derived from microalgae have generated significant interest from leaders in academia, government, and industry.10 Microalgae are considered a promising feedstock for conversion to liquid fuels due to their ability to capture waste carbon dioxide from industrial flue gas streams,11 high photosynthetic yield and lipid content,12 ability to be grown on marginal and non-arable land,13 and potential for achieving policy mandated volumetric renewable fuel targets aimed at mitigating anthropogenic derived climate change and increasing U.S. energy independence and security.In recent years, life-cycle assessment (LCA) has emerged as the preferential method for modeling the energy and environmental performance of biomass-to-fuel systems, and has been extensively applied to emerging microalgal biofuel and bioenergy systems.14–22 Existing LCA of microalgae biofuel production have focused on quantifying the life-cycle greenhouse gas (GHG) emissions and life cycle energy balance for different microalgal growth configurations and fuel conversion pathways.23–30 Additionally, prior work has investigated the impact of microalgal biofuels on nutrient and water resources,31–33 and quantified the geospatial constraints and related impacts on microalgal fuel production.34–37 However, traditional energy analysis suffers from several limitations such as ignoring the difference in quality and substitutability of different resources, and accounting for only the first law of thermodynamics.38–42 Hierarchical thermodynamic based approaches and metrics have been suggested to address the limitations of traditional energy analysis while concurrently providing a methodological framework for quantifying ecological resource consumption from a life-cycle perspective.43–46 However, these have not yet been applied to study emerging microalgal biofuels. Additionally, existing sustainability assessments have ignored the contribution of ecological goods and services (EGS) or natural capital within the algal biofuel supply chain. Natural capital extends the economic notion of capital to include goods and services provided by ecosystems and the natural environment, which are essential to sustaining human and ecological life—such as: timber, food, water, energy resources, clean air, minerals and ores, purification of air and water resources, flood and drought mitigation, pollination of crops and vegetation, maintenance of global biodiversity, as well as climate and disease regulation. Despite the critical importance of EGS to human and global welfare, most existing measures of sustainability do not account for the role and/or consumption of EGS within product or fuel life cycles.39,43,44
Ecological goods and services
Pre-industrial revolution, the paradigm of environmental awareness operated under the assumption that the global ecosphere would be able to absorb the totality of anthropogenic-derived pollution and resource degradation without widespread negative consequences for human life or the environment. However, in recent decades research has reported accelerated degradation of numerous ecosystem goods and services as a direct consequence of economic development and human activity.47 There is increasing realization that anthropogenic-derived environmental impacts can cause irreparable damage to the world's ecosystems and strain the natural ecological functions that support human life.48In 2001, the United Nations (UN) initiated the millennium ecosystem assessment (MEA)—an international collaboration designed to assess the impact and widespread consequences of environmental change for human and ecological well-being. The MEA developed a scientifically rigorous framework for assessing the impacts of environmental change in coupled dynamic socio-ecological systems, and provided guidelines for policy measures and human action required for the conservation and long-term sustainability of the earths biosphere.49 Published in 2005, the findings of the MEA indicate that in the second half of the 20th century anthropogenic-derived resource degradation and overconsumption of natural capital have changed ecosystems more rapidly and extensively than in any comparable period in human history.49 Results from the MEA study indicate that 6 out of the 11 global ecological provisioning services, 7 out of the 10 ecological regulating services, and 2 out of the examined 3 ecological cultural services have been severely degraded over the past 50 years. Furthermore, the results of the MEA indicate that anthropogenic derived environmental impacts have resulted in loss of global biodiversity, and may impair the ability of the planet's ecosystems to sustain human life. Clearly, it is imperative that sustainability assessments consider the consumption of ecological goods and services within biofuel life cycles at early stages of research and development, so as to avoid or mitigate any potential widespread negative impacts for human and global ecological welfare that may result as a consequence of full-scale commercialization of these fuels.
2. Hybrid ecologically based LCA methodology and metrics
EcoLCA is an environmentally extended input–output model capable of accounting for the consumption/role of ecosystem goods and services in a life cycle framework.38,41,50 This work extends the EcoLCA framework developed by Bakshi and colleagues to study the environmental sustainability of emerging microalgal biofuel systems.43,44 The hybrid framework developed in this study utilizes the 2002 EcoLCA model to quantify ecosystem/economy wide impacts, while a detail process inventory is used to assess direct material, energy, and ecological impacts of biofuel production. By integrating process and EcoLCA models, we quantify the total life cycle impacts of microalgal biofuel production. Fig. 1 presents an overview of the hybrid EcoLCA framework utilized in the present study.In this work, we develop a hybrid EcoLCA model to quantify the contribution of ecological resources within the algae-to-fuel life cycle, and to compare the resource intensity of producing microalgal derived renewable diesel and biodiesel to that of traditional petroleum diesel. Furthermore, a host of hierarchical thermodynamic metrics is utilized to address the shortcomings of existing life cycle energy analysis and provide novel insights into the environmental performance and sustainability of emerging microalgal biofuel systems. Renewable diesel also known as “green diesel” is an infrastructure compatible biofuel produced via hydrotreating of algal lipids. RD is attractive as a fuel product because of its high energy density, cetane number, and storage stability.51 Furthermore, RD is fungible with petroleum diesel and as such can be used in current commercial vehicle fleets and fuel infrastructure. The main paper provides an in-depth analysis of microalgal derived RD; results for microalgal biodiesel are provided in the ESI.†
Process model
A detailed process model was developed to quantify the direct material, energy, and ecological inputs for producing algal derived renewable diesel and biodiesel via a theoretical integrated open raceway pond (ORP) biorefinery operating in Phoenix, AZ.23,34 Two technological routes, baseline and improved scenarios, spanning a large feasibility space were evaluated for biofuel production. Baseline scenarios represent current commercial day refining and processing technologies, while improved scenarios represent technological options which have undergone pilot scale experimentation but whose feasibility on a commercial scale has yet to be determined.Algal growth rates were developed based on monthly average meteorological and climate parameters obtained from the National Solar Radiation Database (NSRD)52 and National Oceanic and Atmospheric Administration (NOAA)53 as well as biomass composition and photosynthetic efficiency terms.12,34,54 The fractional composition of the algal biomass was assumed to be 25% lipids (L), 28% carbohydrates (C), and 47% proteins (P).54 The molecular composition of the biomass fractions was constructed based on the work of Lardon et al. 2009.15 It was assumed that the integrated ORP biorefinery operates for eight months out of the calendar year. Furthermore, the biorefinery is constructed on a 1000 hectare plot of land in which 500 hectares are allocated for open raceways ponds and 500 hectares for infrastructure requirements.
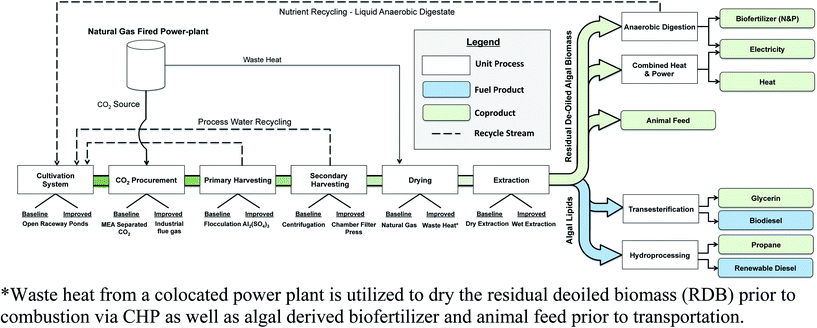
The modeled biomass-to-biofuel production chain consists of the following sub-modules: cultivation, CO2 procurement, primary harvesting, secondary harvesting, drying, lipid extraction, coproduct and fuel conversion, and transportation. An overview of the biomass-to-fuel process chain along with technological routes examined in this work is provided in Fig. 2.
It was assumed that the algal biorefinery would be co-located with a natural gas (NG) fired power plant, which would provide industrial flue gas as a carbon source for algal growth. In the biomass-to-biofuel model, two process options are evaluated for CO2 procurement. In the baseline scenario(s) industrial flue gas is separated into pure CO2 via monoethanolamine scrubbing, this pure CO2 is then compressed and injected into the algae ponds. In the improved scenario(s) industrial flue gas is directly compressed from the NG fired powerplant and pumped into the ORP via low-pressure blowers. Process energy requirements for flue gas/CO2 compression and transportation were constructed based on the work of Kadam 2002.55 While the use of industrial flue gas may reduce the high resource and energy demands associated with CO2 procurement, there is high technological uncertainty regarding the feasibility of direct injection of industrial flue gas and its effects on the microalgal culture.56–58
Inputs for the cultivation of microalgae include: sunlight, freshwater, high density polyethylene (HDPE) pond liner, urea (N-fertilizer), super phosphate (P-fertilizer), potassium chloride (K-fertilizer), industrial flue gas, and electricity required for pumping and water movement as well as for the compression and transportation of industrial flue gas/CO2. It was assumed that a HDPE pond liner would be used to line the ORP,32 and paddlewheels are utilized for circulating the algal growth medium. Energy requirements for sourcing ground/surface water were developed based on the 2008 Farm and Ranch Irrigation Survey.59 Energy requirements for circulation of pond medium and pumping between various system components was developed based on the Darcy–Weisbach equation. Additionally, factors such as water loss due to evaporation, pond leaking, pond blowdown, as well as the embodied impacts of the HDPE pond liner are also considered in the algae-to-fuel model.34
After cultivation, the algal biomass undergoes chemical flocculation via the addition of aluminum sulfate. In the baseline scenario the flocculated algae is further dewatered via a decanter centrifuge, resulting in a solids content of 22% (w/w). In the improved scenarios chamber filter presses are utilized as a means of dewatering the biomass; resulting in a final solids content of 25% weight by weight (w/w). It was assumed that the environmental impacts for the membrane replacement/regeneration are negligible as compared to operating costs, and thus was not considered in the analysis. Process requirements for the harvesting stage were developed based on prior pilot scale tests as well as peer-reviewed and technical literature.60–62 Post harvesting, in the base-case scenario thermal dewatering via combustion of natural gas is performed to dry the algal slurry to a 90% solids content.15 Hexane extraction is then utilized to separate the non-lipid and lipid fraction(s) of the algal biomass. In the improved scenario(s), liquid–liquid (wet) extraction via countercurrent circulation of n-hexane is utilized to separate the lipid and non-lipid fractions of the biomass.23,63 In both technological routes, the extracted lipids are either hydrotreated using hydrogen to produce algal derived renewable diesel as well as coproduct propane or transesterified to produce algal biodiesel and coproduct glycerin. Three process options are considered for the non-lipid fraction of the algal biomass: (1) use as an animal feed, (2) anaerobic digestion of residual de-oiled biomass (RDB) to produce bioelectricity as well as biofertilizer, and (3) cogeneration of RDB via combined heat and power (CHP) to produce bioelectricity and heat. Efficiencies were considered at each stage in the algae-to-fuel process chain including: 75% nutrient uptake efficiency,34 70% CO2 utilization efficiency,29 5% harvesting product loss for centrifugation and chamber filter presses,30 90% process medium recycling for 1st and 2nd stage harvesting,31 95% lipid extraction and conversion efficiency for wet extraction pathways63 and 97% lipid extraction and conversion efficiency for dry extraction pathways,64 5% nitrogen volatilization for recycling of liquid anaerobic digestate,63 and a 25% electrical and 56.3% heat conversion efficiency for combustion of RDB in a combined heat and power plant.65 Detailed process level inventory and model parameters are provided in the ESI.†
EcoLCA model
The EcoLCA model is constructed based on the 2002 input–output (IO) model of the U.S. economy. Input–output models, first developed by Nobel laureate Wassily Leontief, provide a mathematical framework for quantifying the inter-industry transactions between different sectors in an economy or a region. The EcoLCA model extends the IO framework to quantify the direct and indirect environmental impacts that result from economic activities via translating the monetary flows of purchased inputs from the economy to ecological and natural resource consumption, emissions, land-use, and other environmental impact categories via the use of monetary to resource use or emission ratios for industrial sectors.66 As such, information regarding sector-wide economic activity is required to run the EcoLCA model. The economic activity for specific industrial sectors was calculated by translating the resource flows as developed in the process inventory with their corresponding 2002 producer price and aggregating the results. If the 2002 producer price was not available a price inflator was used to convert to the 2002 price equivalent.67 Additionally, EcoLCA does not consider the use phase of purchased inputs from the economy as well as direct ecological good and services that are consumed at the process scale.38 Therefore, economy wide environmental impacts obtained from EcoLCA must be integrated with environmental burdens at the process scale to obtain data on a life-cycle basis. Price data for inputs, as well as detailed material and energy flows are provided in the ESI.†Thermodynamic return on investment
A host of metrics have been utilized in LCA to quantify and compare the energy intensity of producing transportation fuel(s) from petroleum and biomass feedstocks.68–70 Traditional energy metrics, such as energy return on investment (EROI), compare the quantity of primary energy required to produce a functional unit of transportation fuel energy—evaluated over the life cycle.71–73 As the primary function of biofuels lies in their utility to displace petroleum-derived liquid fuels, a fossil-based EROI metric is relevant for measuring and benchmarking the performance and sustainability of emerging microalgal biofuel systems. In this study, EROIfossil is defined as the ratio of output fuel energy to the non-renewable primary energy required for its production, and is provided in eqn (1):
 | (1) |
Common energy metrics such as EROI and variants are often used due to their intuitive appeal and ease of comparison with existing studies. However, these traditional energy metrics implicitly assume that all forms of primary energy are fungible, i.e. the heating value of different primary energy resources such as crude oil, coal, natural gas, uranium, solar, wind, tidal, biomass, and geothermal are perfectly substitutable, have the same work-potential, and thus may be added together and represented via a single aggregate metric.74 This traditional aggregation of different primary energy sources has several limitations including: (1) accounting for only the first law of thermodynamics, (2) assuming perfect substitutability between resources, and (3) failing to account for resource quality; and thus has led some researchers to question the utility of the resulting metrics and their ability for informing decision making.74
Exergy analysis has been proposed and utilized to address some of these shortcomings.40 Exergy represents the maximum amount of useful work that can be extracted from a system (or resource) when it is brought into thermodynamic equilibrium with the surrounding environment or reference state.40 The presence of exergy destruction in a system indicates the possibility of a thermodynamic improvement; thus exergy analysis has been widely used in process engineering for optimizing industrial operations and commercial processes.40 Exergy is appealing from a methodological standpoint since it considers both the first and second law of thermodynamics, and can aggregate material and energy resources using a common denominator (joules). As such, aggregating primary energy sources based on exergy provides a better representation of the ability of these resources to produce useful work—as compared to energy. The quantity of exergy consumed throughout the industrial supply chain is defined as the Industrial Cumulative Exergy Consumption (ICEC). Analogous to EROIfossil, the fossil exergy return on investment (ExROIfossil) for a fuel is defined as the ratio of output exergy of the fuel product to the non-renewable ICEC required for its production and is provided in eqn (2):
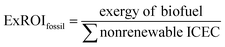 | (2) |
While traditional exergy analysis overcomes many of the shortcomings of energy analysis, it does not account for the quality of different energy or material resources, nor does it consider the contribution of ecosystems in making ecological goods and services available for human and industrial activities. As natural capital provides the basis for human made capital, quantifying the consumption of ecological goods and services across the life cycle is critical for determining the sustainability of emerging microalgal biofuels.
Odum developed a methodological framework built upon principles and concepts from thermodynamics, general systems theory, and systems ecology to understand the dynamic transformation of energy and resource flows within human-ecological systems, in what is formally known today as emergy.75 Odum observed that, in regards to the ‘global energy budget’, incident solar exergy becomes concentrated in as it flows through human-ecological systems. Analogous to energy pyramids commonly used in traditional ecological and food-chain modeling, emergy analysis posits that a hierarchical energy structure exists in human-ecological systems in which dilute sunlight is converted to plant matter, from plant matter to coal, from coal to oil, to electricity and other products, and finally to human made goods and services.76 Therefore, the utility of a resource as well as the ability of an energy carrier to provide useful work are measured in respect to both quantity (MJ, kW h, BTU, kg, etc.) and quality—the amount of available energy of one kind of a lower grade required to develop the higher grade.76 Emergy is formally defined as “the amount of available energy of one kind that is used up in transformations directly and indirectly to generate a product or service”, and is typically expressed in terms of solar equivalent joules (seJ).75 Emergy analysis provides an objective basis for comparing energy and material resources by assessing the direct and indirect past solar energy required for their production. The ratio of emergy input to exergy output of a product or service is defined as transformity, expressed in eqn (3)
 | (3) |
By definition transformity evaluates the amount of emergy required to create a unit of available energy (exergy) of another form. As such, transformity provides a quantitative measure for determining and ranking the quality of different energy and material resources. The amount of past solar exergy that is consumed throughout the ecological and industrial supply chain is referred to as ecological cumulative exergy consumption (ECEC), and is equivalent to emergy if the same system boundary and accounting procedures are chosen.39 Furthermore, a fuels emergy return on investment (EmROI), analogous to the prior return on investment (ROI) metrics, is defined below in eqn (4):
 | (4) |
While the emergy methodology has distinct advantages over traditional energy and exergy analysis, uncertainty in reported transformity values and misperception regarding emergy accounting has limited its widespread adoption.77 Nevertheless, emergy intrinsically considers differences in the quality and substitutability of resources, and provides a consistent, scientifically rigorous, and eco-centric framework for valuing the contribution of ecological processes and natural capital.78 However, as energy, exergy, and emergy analysis all offer unique insights regarding the sustainability of a product or service, a hierarchy of sustainability and performance indicators based on these thermodynamic metrics may be preferable.
Thermodynamic based sustainability metrics
In this work a variety of thermodynamic return on investment metrics, sustainability indicators, and renewability indices based off of energy, exergy and emergy analysis are used to quantify the consumption of ecological goods and services, environmental impacts, and resource intensity of producing microalgal derived fuels. Several performance metrics based on ECEC analysis including: ECEC Yield Ratio (EYR), Environmental Loading Ratio (ELR), Yield-to-Loading Ratio (YLR), and Renewability Index (R) are used to assess the sustainability of transportation fuel production and are formally defined and summarized in Table 1.3. Results and discussion
Thermodynamic return on investment
Fig. 3 presents thermodynamic return on investment metrics for the production of microalgal RD via current day commercial technologies (baseline scenarios) and optimistic future technologies (improved scenarios) under several coproduct scenarios, and compares the results with petroleum diesel. For EROIfossil, ExROIfossil, or EmROI a value greater than one is desirable as it indicates that more work is produced per functional unit via the fuel system relative to the work invested for its production. Comparison of these thermodynamic metrics across multiple microalgal biofuel processing technologies and coproduct options provides unique perspectives on the performance and environmental sustainability of current and future microalgal biofuel systems at both the industrial and coupled industrial-ecological scale.The results reveal that current day microalgal RD production has a low return on investment (ROI < 1) for all examined thermodynamic metrics and coproduct scenarios, and is a consequence of the exceedingly resource intensive stages in the algae-to-fuel process chain: including the high energy requirements for water circulation and pumping in the ORP system,32 dewatering and harvesting operations,79 high upstream impacts of synthetic fertilizers,80 CO2 procurement,34 and drying. Future and optimistic processing technologies (improved scenarios) offer superior ROI but are plagued with high technological uncertainty as these scenarios have yet to be effectively demonstrated at a commercial scale. Error bars for baseline and improved scenarios represent the results for high (40% L/50% C/10% P) and low lipid (10% L/20% C/70% P) biomass composition, while error bars for petroleum diesel represent the range obtained via market and mass based allocation. The results from Fig. 3 indicate only one of the evaluated RD production pathways yields an EROIfossil greater than one (improved scenarios utilizing CHP). However, analysis reveals that by correcting for the availability of the energy (i.e. ExROIfossil) the resulting ROI is less than unity. This value is further lowered when correcting for the quality of resources (i.e. EmROI). These results are compelling as they suggest that after accounting for the quality of resources, more useful work is invested via the economy in producing microalgal fuels than useful work generated from these fuels.
Although traditional energy metrics such as EROI are commonly used in the fuel and energy literature, this work has shown that these metrics fail to accurately measure the amount of useful work generated via microalgal biofuel systems as well as adequately quantify the amount of past ecological work required for their production, thus producing misleading and erroneous results regarding the environmental sustainability and performance of these fuel systems. These findings are significant, as traditional energy metrics such as EROI are frequently utilized to guide the course of fuel and energy policy and the sustainable adoption of fuel and energy resources.
The results from Fig. 3 reveal that petroleum diesel is thermodynamically superior to microalgal RD as indicated by its high ROI across all thermodynamic metrics relative to microalgal fuels. The high ROI for petroleum diesel is a direct consequence of the fact that nature has performed most of the past work in making these resources available for human consumption. The low ROI for microalgae biofuels reflects the highly engineered and resource intensive nature of microalgal fuel production and has broad sustainability implications, as the large scale adoption of alternative fuels with lower ROI relative to petroleum transportation fuels could have long-standing societal consequences81,82 as a greater portion of useful work must be diverted from the economy for fuel production and thus cannot be used to sustain other economic activities.
Fig. 4 plots the fractional contribution of energy, ICEC, and ECEC by ecological resource type for microalgal RD production under different coproduct options and compares the results to petroleum diesel. Fig. 4 shows that natural gas, coal, sunlight, and crude oil contribute the majority of total energy consumption in the algae-to-fuel supply chain, with similar trends found across the evaluated coproduct scenarios. ICEC analysis expands upon traditional energy analysis to consider both energy and material inputs; this is evident from the contribution of metallic and non-metallic ores, water, and other material inputs to overall resource consumption. However, the contribution of these resources is still small relative to other ecological resources (such as natural gas, coal, etc.). As shown in Fig. 4, the contribution of low-quality resources such as sunlight have a significant impact on overall resource contribution in traditional energy and exergy analysis. This is a direct consequence of the fact that low quality resources are weighted equitably with other energy and natural resources in traditional energy and exergy analysis. However, emergy analysis corrects for the quality of resources by comparing them in terms of their solar equivalence. As such, their contribution is minimal when evaluated from an emergy perspective. Furthermore, resources of higher quality (high transformity) such as metallic and non-metallic ores are found to comprise a larger fraction of total ecological resource contribution when evaluated via ECEC analysis. The results from Fig. 4 show that crude oil constitutes over 85% of total resource consumption in energy, ICEC, and ECEC analysis of petroleum diesel production. As such, petroleum diesel requires less work from the economy for conversion to transportation fuel compared to microalgal biofuels, as the majority of past work has been provided by nature via the formation of crude oil. Consequently, this results in a higher ROI for petroleum diesel relative to microalgal renewable diesel.
 | ||
| Fig. 4 Fractional contribution of total energy, ICEC, ECEC by ecological resource for microalgal renewable diesel and petroleum diesel. | ||
Table 2 provides an overview of the ECEC sustainability and thermodynamic performance metrics for the scenarios considered in this work. The results show that petroleum diesel has a high ECEC yield ratio as a larger amount of past work has been performed by nature in producing crude oil as compared to the direct and indirect solar exergy of purchased inputs from the economy required for its extraction, refining, etc. However, microalgal biofuels require substantial material and energy inputs from the economy, and a comparatively minimal amount of past work from direct ecological good and services (direct sunlight, freshwater, and photosynthetic CO2). As such, the EYR for petroleum diesel is larger than that of microalgal derived renewable diesel. The results from Table 2 reveal that petroleum diesel has a low renewability index and low YLR, indicating that these fuels are not sustainable in the long-term. Additionally, the results indicate that production of microalgal biofuels is highly dependent on non-renewable ecological resources reflected in the low renewability index and YLR, and high ELR. The high ELR for microalgal derived RD (ELR > 1 for all examined production pathways) indicates that more non-renewable ECEC is utilized in the algal-to-fuel supply chain as compared to renewable ECEC. Additional results for microalgal-derived biodiesel are provided in the ESI.†
| Transportation fuel | Petroleum diesel | Microalgal RD (baseline) | Microalgal RD (improved) | ||||
|---|---|---|---|---|---|---|---|
| a AF = animal feed; AD = anaerobic digestion; CHP = combined heat and power. | |||||||
| Coproduct scenarios | N/A | AF | AD | CHP | AF | AD | CHP |
| Renewability index (%) | 0.13 | 3.62 | 3.51 | 3.63 | 6.34 | 6.23 | 6.36 |
| ECEC yield ratio | 6.34 | 1.02 | 1.02 | 1.02 | 1.04 | 1.04 | 1.04 |
| Environmental loading ratio | 760.19 | 26.62 | 27.53 | 26.57 | 14.78 | 15.06 | 14.72 |
| Yield-to-load ratio | 0.01 | 0.04 | 0.04 | 0.04 | 0.07 | 0.07 | 0.07 |
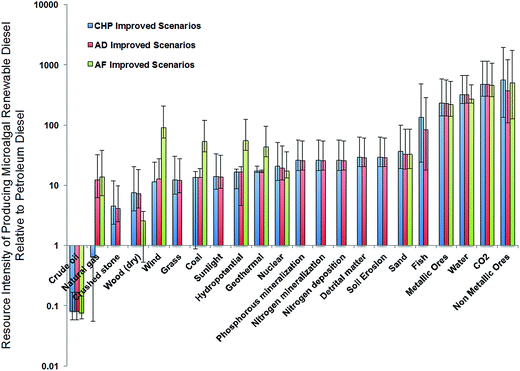
Fig. 5 plots the ratio of ecological resource intensity of producing microalgal renewable diesel relative to petroleum diesel for a common functional unit, 1 megajoule (MJ). The results indicate that microalgal RD consumes significantly more metallic/non-metallic ores and water resources, and generally have higher ecological resource intensity as compared to petroleum diesel on a functional unit basis. For animal feed pathways microalgal RD provides benefits in the following ecological resources categories: crude oil, crushed ore, grass, sunlight, phosphorus and nitrogen mineralization, nitrogen deposition, detrital matter, soil erosion, and fish relative to petroleum diesel. For anaerobic (AD) and combined heat and power (CHP) coproduct scenarios, microalgal derived renewable diesel has higher ecological resource consumption relative to petroleum diesel across all resource categories except for crude oil and natural gas. These findings are startling and suggest that the large-scale adoption of microalgae biofuels could result in heightened ecosystem degradation, potentially negating the environmental benefits of these fuels. It is important to note that ECEC analysis considers energy and resource flows in complex coupled industrial-ecological systems, and thus has a high degree of uncertainty relative to traditional energy and exergy analysis. Coupling the results obtained via EcoLCA with dynamic ecological modeling83 can provide a spatially and temporally explicit framework for quantifying the contribution of ecosystems goods, and potentially reduce uncertainty in the quantification of direct ecological goods and services. However, such an analysis is beyond the scope of this present study. Methods such as economic valuation of natural capital provide an alternative basis for valuing the contribution of EGS.84 However, economic valuation is sensitive to market distortions and price volatility, and may not capture environmental externalities. Furthermore, economic valuation intrinsically considers the value added via a service or resource in regards to its utility for mankind. This anthro-centric framework is diametrically opposed to the eco-centric framework utilized in ECEC or emergy analysis.
Two strategies exist to enhance the performance and sustainability of microalgal biofuels: (1) reduce the amount of purchased inputs from the economy required for microalgal fuel production, or (2) leverage natural ecological processes to increase the amount of past work provided by nature in making microalgal biofuels available for human consumption. The first option can be met with increases in technological maturation and commercial optimization of algae-to-fuel conversion processes. Multiple strategies such as genetic modification,85 hydrothermal liquefaction,86,87 cross flow and membrane based filtration/separations,88 and industrial symbiosis89 via the use of wastewater90,91 and other synergies are being explored for increasing the performance of emerging microalgal biofuel systems. However, it is important to recognize that microalgae biofuel production is ultimately constrained via the 2nd law efficiency, i.e. the minimum thermodynamic work required for fuel production.92 For the second option, it is possible to envision a scenario in which microalgae are consumed via predators at a higher trophic level in the ecological food chain (such as fish). Assuming that the lipid fraction of the microalgae be absorbed and retained via these predators, it may be possible to harvest and utilize these organisms for conversion to liquid transportation fuels,93 effectively allowing nature to perform the work traditionally required via energy intensive dewatering and conversion processes. Additionally, alternate microalgal processing options such as solar drying of the biomass may reduce the amount of human made work required for biofuel production. However, the high land-use requirements for solar drying may limit its commercial applicability.
4. Conclusions
Failure to consider the impacts of emerging technologies on ecological goods and services before their widespread adoption and use could result in unsustainable choices and dramatic consequences for the earth's ecosphere including heightened depletion and degradation of the global ecological resource base, potentially straining the ecological functions that support human life. Thermodynamic analysis based on exergy and emergy provides a scientifically rigorous approach for valuing the contribution of ecological goods and services in product life cycles, and concurrently addresses several existing problems in traditional energy analysis including accounting for the quality, substitutability, and useful work provided via material and energy resources. This study highlights the fallacies of traditional energy analysis, and shows that exergy and emergy analysis can provide valuable insights into the sustainability and performance of emerging microalgal biofuel systems. This work has shown that in the best-case scenario microalgal fuel systems are marginally energy positive, and more ecologically resource intensive as compared to their petroleum equivalent on a functional unit basis. However, technological maturation/optimization of the algae-to-fuel production chain as well as coupling microaglal biofuel production with ecological processes have the potential for reducing the amount of human made work required for biofuel production while concurrently increasing the sustainability of these emerging fuel systems. The hierarchical thermodynamic-based resource aggregation scheme utilized in this work can be extended to other nascent fuel and energy platforms and thus help guide the sustainable development and adoption of next-generation biofuels.Appendix
Abbreviations
| AD | Anaerobic digestion |
| AF | Animal feed |
| AZ | Arizona |
| C | Carbohydrate |
| CHP | Combined heat and power |
| CO2 | Carbon dioxide |
| ECEC | Ecological cumulative exergy consumption |
| EcoLCA | Ecologically based life cycle assessment |
| EGS | Ecological goods and services |
| ELR | Environmental loading ratio |
| EROI | Energy return on investment |
| ExROI | Exergy return on investment |
| EmROI | Emergy return on investment |
| EYR | ECEC yield ration |
| GHG | Greenhouse gas emissions |
| HDPE | High density polyethylene |
| ICEC | Industrial cumulative exergy consumption |
| IO | Input–output |
| L | Lipid |
| LCA | Life cycle assessment |
| MEA | Millennium ecosystems assessment |
| MJ | Megajoule |
| NG | Natural gas |
| NOAA | National Oceanic and Atmospheric Administration |
| NSRD | National Solar Radiation Database |
| ORP | Open raceway pond |
| P | Protein |
| PD | Petroleum diesel |
| R | Renewability index% |
| RDB | Residual de-oiled biomass |
| RD | Renewable diesel |
| ROI | Return on investment |
| ESI | Electronic supporting information |
| UN | United Nations |
| US | United States |
| YLR | Yield to loading ratio |
References
- F. Sissine, Energy Independence and Security Act of 2007: A Summary of Major Provisions, Congressional Research Service Report for Congress, Order Code RL34294, Washington, DC, 2007 Search PubMed.
- D. Tilman, R. Socolow, J. A. Foley, J. Hill, E. Larson, L. Lynd, S. Pacala, J. Reilly, T. Searchinger, C. Somerville and R. Williams, Science, 2009, 325, 270–271 CrossRef CAS PubMed.
- S. Nonhebel, Energy, 2012, 37, 115–121 CrossRef PubMed.
- J. Fargione, J. Hill, D. Tilman, S. Polasky and P. Hawthorne, Science, 2008, 319, 1235–1238 CrossRef CAS PubMed.
- J. M. Melillo, J. M. Reilly, D. W. Kicklighter, A. C. Gurgel, T. W. Cronin, S. Paltsev, B. S. Felzer, X. D. Wang, A. P. Sokolov and C. A. Schlosser, Science, 2009, 326, 1397–1399 CrossRef CAS PubMed.
- T. Searchinger, R. Heimlich, R. A. Houghton, F. X. Dong, A. Elobeid, J. Fabiosa, S. Tokgoz, D. Hayes and T. H. Yu, Science, 2008, 319, 1238–1240 CrossRef CAS PubMed.
- T. E. McKone, W. W. Nazaroff, P. Berck, M. Auffhammer, T. Lipman, M. S. Torn, E. Masanet, A. Lobscheid, N. Santero, U. Mishra, A. Barrett, M. Bomberg, K. Fingerman, C. Scown, B. Strogen and A. Horvath, Environ. Sci. Technol., 2011, 45, 1751–1756 CrossRef CAS PubMed.
- R. Sims, M. Taylor, J. Saddler and W. Mabee, International Energy Agency and Organization for Economic Cooperation and Development, 2008.
- D. Pimentel, T. Patzek and G. Cecil in Reviews of Environmental Contamination and Toxicology, ed. D. Whitacre, G. Ware, H. Nigg, D. Doerge, L. Albert, P. Voogt, C. Gerba, O. Hutzinger, J. Knaak, F. Mayer, D. P. Morgan, D. Park, R. Tjeerdema, R. H. Yang and F. Gunther, Springer New York, 2007, ch. 2, vol. 189, pp. 25–41 Search PubMed.
- U. S. DOE, National Algal Biofuels Technology Roadmap, U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, BIomass Program, 2010 Search PubMed.
- D. Brune, T. Lundquist and J. Benemann, J. Environ. Eng., 2009, 135, 1136–1144 CrossRef CAS.
- K. M. Weyer, D. R. Bush, A. Darzins and B. D. Willson, BioEnergy Res., 2010, 3, 204–213 CrossRef.
- Y. Chisti, Biotechnol. Adv., 2007, 25, 13 CrossRef PubMed.
- R. Chowdhury, S. Viamajala and R. Gerlach, Bioresour. Technol., 2012, 108, 102–111 CrossRef CAS PubMed.
- L. Lardon, A. Hélias, B. Sialve, J.-P. Steyer and O. Bernard, Environ. Sci. Technol., 2009, 43, 6475–6481 CrossRef CAS.
- T. Shirvani, X. Yan, O. R. Inderwildi, P. P. Edwards and D. A. King, Environ. Sci. Technol., 2011, 4, 3773–3778 CAS.
- P. K. Campbell, T. Beer and D. Batten, Bioresour. Technol., 2011, 102, 50–56 CrossRef CAS PubMed.
- R. M. Handler, D. R. Shonnard, T. N. Kalnes and F. S. Lupton, Algal Res., 2014, 4, 105–115 CrossRef PubMed.
- K. Sander and G. Murthy, Int. J. Life Cycle Assess., 2010, 15, 704–714 CrossRef CAS PubMed.
- K. Cox, M. Renouf, A. Dargan, C. Turner and D. Klein-Marcuschamer, Biofuels, Bioprod. Biorefin., 2014, 8, 579–593 CrossRef CAS.
- L. Yanfen, H. Zehao and M. Xiaoqian, Energy Policy, 2012, 45, 142–151 CrossRef PubMed.
- D. L. Sills, V. Paramita, M. J. Franke, M. C. Johnson, T. M. Akabas, C. H. Greene and J. W. Tester, Environ. Sci. Technol., 2012, 47, 687–694 CrossRef PubMed.
- G. G. Zaimes and V. Khanna, Environ. Prog. Sustainable Energy, 2013, 32, 926–936 CrossRef CAS.
- L. B. Brentner, M. J. Eckelman and J. B. Zimmerman, Environ. Sci. Technol., 2011, 45, 7060–7067 CrossRef CAS PubMed.
- A. F. Clarens, H. Nassau, E. P. Resurreccion, M. A. White and L. M. Colosi, Environ. Sci. Technol., 2011, 45, 7554–7560 CrossRef CAS PubMed.
- L. Batan, J. Quinn, B. Willson and T. Bradley, Environ. Sci. Technol., 2010, 44, 7975–7980 CrossRef CAS PubMed.
- L. F. Razon and R. R. Tan, Appl. Energy, 2011, 88, 3507–3514 CrossRef CAS PubMed.
- O. Jorquera, A. Kiperstok, E. A. Sales, M. Embiruçu and M. L. Ghirardi, Bioresour. Technol., 2010, 101, 1406–1413 CrossRef CAS PubMed.
- A. L. Stephenson, E. Kazamia, J. S. Dennis, C. J. Howe, S. A. Scott and A. G. Smith, Energy Fuels, 2010, 24, 4062–4077 CrossRef CAS.
- V. Vasudevan, R. W. Stratton, M. N. Pearlson, G. R. Jersey, A. G. Beyene, J. C. Weissman, M. Rubino and J. I. Hileman, Environ. Sci. Technol., 2012, 46, 2451–2459 CrossRef CAS PubMed.
- J. Yang, M. Xu, X. Z. Zhang, Q. A. Hu, M. Sommerfeld and Y. S. Chen, Bioresour. Technol., 2011, 102, 159–165 CrossRef CAS PubMed.
- C. F. Murphy and D. T. Allen, Environ. Sci. Technol., 2011, 45, 5861–5868 CrossRef CAS PubMed.
- L. Batan, J. C. Quinn and T. H. Bradley, Algal Res., 2013, 2, 196–203 CrossRef PubMed.
- G. Zaimes and V. Khanna, Biotechnol. Biofuels, 2013, 6, 88 CrossRef CAS PubMed.
- R. E. Davis, D. B. Fishman, E. D. Frank, M. C. Johnson, S. B. Jones, C. M. Kinchin, R. L. Skaggs, E. R. Venteris and M. S. Wigmosta, Environ. Sci. Technol., 2014, 48, 6035–6042 CrossRef CAS PubMed.
- M.-O. P. Fortier and B. S. Sturm, Environ. Sci. Technol., 2012, 46, 11426–11434 CrossRef CAS PubMed.
- E. R. Venteris, R. L. Skaggs, A. M. Coleman and M. S. Wigmosta, Biomass Bioenergy, 2012, 47, 483–497 CrossRef PubMed.
- A. Baral and B. R. Bakshi, Environ. Sci. Technol., 2010, 44, 800–807 CrossRef CAS PubMed.
- J. L. Hau and B. R. Bakshi, Environ. Sci. Technol., 2004, 38, 3768–3777 CrossRef CAS.
- J. Dewulf, H. Van Langenhove, B. Muys, S. Bruers, B. R. Bakshi, G. F. Grubb, D. M. Paulus and E. Sciubba, Environ. Sci. Technol., 2008, 42, 2221–2232 CrossRef CAS.
- A. Baral, B. R. Bakshi and R. L. Smith, Environ. Sci. Technol., 2012, 46, 2436–2444 CrossRef CAS PubMed.
- J. Dewulf, H. Van Langenhove and B. Van De Velde, Environ. Sci. Technol., 2005, 39, 3878–3882 CrossRef CAS.
- Y. Zhang, A. Baral and B. R. Bakshi, Environ. Sci. Technol., 2010, 44, 2624–2631 CrossRef CAS PubMed.
- Y. Zhang, S. Singh and B. R. Bakshi, Environ. Sci. Technol., 2010, 44, 2232–2242 CrossRef CAS PubMed.
- B. De Meester, J. Dewulf, A. Janssens and H. Van Langenhove, Environ. Sci. Technol., 2006, 40, 6844–6851 CrossRef CAS.
- J. Dewulf, M. E. Bösch, B. D. Meester, G. V. d. Vorst, H. V. Langenhove, S. Hellweg and M. A. J. Huijbregts, Environ. Sci. Technol., 2007, 41, 8477–8483 CrossRef CAS.
- S. R. Carpenter, H. A. Mooney, J. Agard, D. Capistrano, R. S. DeFries, S. Diaz, T. Dietz, A. K. Duraiappah, A. Oteng-Yeboah and H. M. Pereira, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 1305–1312 CrossRef CAS PubMed.
- S. Pimm, C. Jenkins, R. Abell, T. Brooks, J. Gittleman, L. Joppa, P. Raven, C. Roberts and J. Sexton, Science, 2014, 344, 1246752 CrossRef CAS PubMed.
- M. E. Assessment, Ecosystems and Human Well-being: Synthesis, Island Press, 2005 Search PubMed.
- Ecologically-Based Life Cycle Assessment, The Ohio State University, The Center For Resilience, http://resilience.eng.ohio-state.edu/eco-lca/.
- T. Kalnes, T. Marker and D. R. Shonnard, Int. J. Chem. React. Eng., 2007, 5 Search PubMed.
- NREL, National Renewable Energy Laboratory, Golden, CO, 1994.
- NOAA, National Climatic Data Center, National Oceanic and Atmospheric Administration, 2006 Search PubMed.
- P. J. l. B. Williams and L. M. L. Laurens, Energy Environ. Sci., 2010, 3, 554–590 CAS.
- K. L. Kadam, Energy, 2002, 27, 905–922 CrossRef CAS.
- H. Matsumoto, N. Shioji, A. Hamasaki, Y. Ikuta, Y. Fukuda, M. Sato, N. Endo and T. Tsukamoto, Appl. Biochem. Biotechnol., 1995, 51, 681–692 CrossRef.
- M. Negoro, N. Shioji, K. Miyamoto and Y. Micira, Appl. Biochem. Biotechnol., 1991, 28–29, 877–886 CrossRef CAS.
- S. Cho, D. Lee, T. T. Luong, S. Park, Y. K. Oh and T. Lee, J. Microbiol. Biotechnol., 2011, 21, 1073–1080 CrossRef CAS.
- U. NASS, US Department of Agriculture, National Agricultural Statistics Service, 2008.
- F. H. Mohn, Experiences and Strategies in the recovery of biomass from mass cultures of microalgae, Amsterdam, Elsevier, 1980 Search PubMed.
- C. G. Golueke and W. J. Oswald, J.–Water Pollut. Control Fed., 1965, 471–498 Search PubMed.
- G. Shelef, A. Sukenik and M. Green, Microalgae Harvesting and Processing: A Literature Review, U. S. D. o. Energy Report SERI/STR-231–2396, Solar Energy Research Institute, Golden, Colorado, 1984 Search PubMed.
- E. Frank, J. Han, I. Palou-Rivera, A. Elgowainy and M. Wang, Center for Transportation Research, Energy Systems Division, Argonne National Laboratory, Oak Ridge, 2011.
- A. Prabhu, C. Pham, A. Glabe and J. Duffy, Draft.
- H. H. R. Rauch, K. Bosch, I. Siefert, C. Aichernig, H. Tremmel, K. Voigtlaender, R. Koch and R. Lehner, presented in part at the World Conference and Technology Exhibition on Biomass for Energy, Italy Search PubMed.
- N. U. Ukidwe and B. R. Bakshi, Environ. Sci. Technol., 2004, 38, 4810–4827 CrossRef CAS.
- CPI Inflation Calculator, 2013.
- C. J. Cleveland, Net energy analysis, http://www.eoearth.org/article/Net_energy_analysis, accessed July 4 2011 Search PubMed.
- K. Mulder and N. J. Hagens, Ambio, 2008, 37, 74–79 CrossRef.
- L. Xu, D. W. F. Brilman, J. A. M. Withag, G. Brem and S. Kersten, Bioresour. Technol., 2011, 102, 5113–5122 CrossRef CAS PubMed.
- D. J. Murphy and C. A. S. Hall, Ann. N. Y. Acad. Sci., 2010, 1185, 102–118 CrossRef PubMed.
- C. A. Hall, S. Balogh and D. J. Murphy, Energies, 2009, 2, 25–47 CrossRef PubMed.
- R. Hammerschlag, Environ. Sci. Technol., 2006, 40, 1744–1750 CrossRef CAS.
- B. E. Dale, Biofuels, Bioprod. Biorefin., 2007, 1, 14–17 CrossRef CAS.
- H. T. Odum, Environmental Accounting: Emergy and Environmental Decision Making, Wiley, 1996 Search PubMed.
- H. T. Odum, Ambio, 1973, 220–227 Search PubMed.
- M. T. Brown and R. A. Herendeen, Ecol. Econ., 1996, 19, 219–235 CrossRef.
- J. L. Hau and B. R. Bakshi, Ecol. Modell., 2004, 178, 215–225 CrossRef PubMed.
- N. Uduman, Y. Qi, M. K. Danquah, G. M. Forde and A. Hoadley, J. Renewable Sustainable Energy, 2010, 2, 012701–012715 CrossRef PubMed.
- A. F. Clarens, E. P. Resurreccion, M. A. White and L. M. Colosi, Environ. Sci. Technol., 2010, 44, 1813–1819 CrossRef CAS PubMed.
- C. A. S. Hall, J. G. Lambert and S. B. Balogh, Energy Policy, 2014, 64, 141–152 CrossRef PubMed.
- J. G. Lambert, C. A. S. Hall, S. Balogh, A. Gupta and M. Arnold, Energy Policy, 2014, 64, 153–167 CrossRef PubMed.
- A. J. Guswa, K. A. Brauman, C. Brown, P. Hamel, B. L. Keeler and S. S. Sayre, Water Resour. Res., 2014, 50, 4535–4544 CrossRef.
- C. J. Cleveland, R. K. Kaufmann and D. I. Stern, Ecol. Econ., 2000, 32, 301–317 CrossRef.
- R. Radakovits, R. E. Jinkerson, A. Darzins and M. C. Posewitz, Eukaryotic Cell, 2010, 9, 486–501 CrossRef CAS PubMed.
- P. E. Savage, Science, 2012, 338, 1039–1040 CrossRef CAS PubMed.
- M.-O. P. Fortier, G. W. Roberts, S. M. Stagg-Williams and B. S. M. Sturm, Appl. Energy, 2014, 122, 73–82 CrossRef CAS PubMed.
- R. Bhave, T. Kuritz, L. Powell and D. Adcock, Environ. Sci. Technol., 2012, 46, 5599–5606 CrossRef CAS PubMed.
- K. Soratana and A. E. Landis, Bioresour. Technol., 2011, 102, 6892–6901 CrossRef CAS PubMed.
- . B. K. Park, R. J. Craggs and A. N. Shilton, Bioresour. Technol., 2011, 102, 35–42 CrossRef PubMed.
- E. Menger-Krug, J. Niederste-Hollenberg, T. Hillenbrand and H. Hiessl, Environ. Sci. Technol., 2012, 46, 11505–11514 CrossRef CAS PubMed.
- C. M. Beal, R. E. Hebner and M. E. Webber, Energy, 2012, 44, 925–943 CrossRef CAS PubMed.
- V. R. Wiggers, A. Wisniewski Jr, L. A. S. Madureira, A. A. C. Barros and H. F. Meier, Fuel, 2009, 88, 2135–2141 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09191d |
| This journal is © The Royal Society of Chemistry 2014 |