Full picture of the thermotropic phase behavior of cardiolipin bilayer in water: identification of a metastable subgel phase
Fu-Gen Wuab,
Hai-Yuan Sunb,
Yu Zhoub,
Rui-Guang Wuc and
Zhi-Wu Yu*b
aState Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, P. R. China
bKey Laboratory of Bioorganic Phosphorous Chemistry and Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, P. R. China. E-mail: yuzhw@tsinghua.edu.cn
cSchool of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing 100102, P. R. China
First published on 7th October 2014
Abstract
The formation of a stable crystalline phase from a fluid phase usually involves the passage through a gel or metastable subgel phase during cooling. Herein, we found for the first time that for the four-tailed lipid, tetramyristoyl cardiolipin (TMCL), a metastable subgel phase (“subgel II”-type phase, or SGII) was formed upon cooling from the gel phase, and such a metastable subgel phase served as the precursor towards the formation of a stable crystalline phase. A detailed thermotropic, X-ray diffraction and infrared spectroscopic study was carried out to study the formation of the subgel and crystalline phases, along with a comparison of the gel and fluid phases. All the four phases (crystalline, subgel, gel and fluid) are lamellar structured and the interlamellar spacings of the gel and fluid phases swell as the water content increases in pure water. A full picture of the thermotropic phase behavior of TMCL in water was obtained and it was found that the transformations between fluid–gel and gel–subgel are reversible, while the phase transformation processes involving the crystalline phase are irreversible. The gel to subgel transition mainly involves the reorganization of the tail packing state (from hexagonal to orthorhombic), and this phase transition was found to be independent of solvent. Changing from subgel to crystalline phase needs a long incubation time at relatively low temperatures (0–5 °C), and this crystalline phase converts to a gel phase upon heating. The present work sheds light on the crystallization process of amphiphiles and has profound implications on the stability of cardiolipin-based model systems or supramolecular self-assembly systems.
1. Introduction
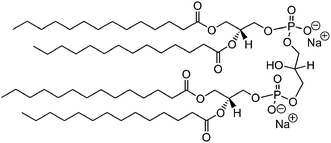
Cardiolipin (CL) is a kind of diphosphatidylglycerol lipid, with its two phosphatidylglycerols connecting with a glycerol backbone in the center to form a dimeric structure with four hydrocarbon chains. Each CL molecule possesses two phosphates, and the ionization of the two phosphates happens at a very different level of acidity: pK1 = 3 and pK2 > 7.5. Thus under normal physiological conditions (pH around 7), the molecule may carry only one negative charge. One of two phosphates may form a stable intramolecular hydrogen bond with the hydroxyl group of the centered glycerol, thus forming a bicyclic resonance structure. This structure traps one proton, which is quite helpful for oxidative phosphorylation.1 CL is an important component of the inner mitochondrial membrane,2 where it constitutes about 20–25% of the total lipid composition. CL is essential for the optimal function of numerous enzymes that are involved in mitochondrial energy metabolism, and thus any physiological or pathological perturbations in its synthetic and catabolic pathways can significantly affect mitochondrial structure and function, and ultimately cell survival.3–5 More specifically, CL has been found to play a key role in the proper function of the mitochondrial heme protein cytochrome c (cyt c).6–11 The level of CL is a critical indicator of numerous diseases associated with mitochondrial respiratory functions. And as a result, a highly selective and sensitive detection method for cardiolipin has been developed very recently.12 Besides, CL is the major lipid component in most Gram-positive bacteria, and it can also be found in Gram negative bacteria.13,14 Further, because of its unique size (i.e. its head-to-tail ratio), CL can be used to modulate the curvature of a synthetic model membrane, which has wide implications for supramolecular chemistry and colloidal and interface science. With the presence of divalent metals, CL becomes a non-bilayer forming lipid and can have membrane fusion and lamellar to inverted-hexagonal phase transition.15CL has been employed as a key component in various natural or synthetic biological membranes; however, the detailed phase structure and phase behavior of this unique molecule have been seldomly studied.16 Lewis and co-workers have reported the thermotropic phase behavior of CL bilayers and found that there are three lamellar phases (fluid, gel and subgel) and two phase transitions (subgel to gel, gel to fluid) upon heating.16 Here the subgel phase is actually a stable lamellar crystalline phase. However, a complete picture of the thermotropic phase behavior of CL is still lacking, and the formation mechanisms of the crystalline phase needs a more detailed investigation. In this work, by using differential scanning calorimetry (DSC), synchrotron small- and wide-angle X-ray scatterings (SAXS and WAXS) and Fourier transform infrared (FTIR) spectroscopy, we carefully characterized the phase structures and molecular transition mechanisms of tetramyristoyl diphosphatidylglycerol or tetramyristoyl cardiolipin (TMCL, see its molecular structure in Fig. 1) bilayer in water. A new metastable subgel phase (“subgel II” type phase, SGII phase) was identified for the first time, and a complete phase transformation process for TMCL in water has been uncovered. The conversion kinetics of the stable crystalline phase (“subgel I” type phase) was examined by DSC and it was found that a complete formation of the crystalline phase requires the incubation of the sample at 0–5 °C for more than two weeks; while we found that the metastable subgel phase was formed by cooling the gel phase to a temperature below 10 °C, and this phase transition only involves a significant ordering of the lipid hydrocarbon tails.
2. Experimental section
2.1. Sample preparation
Tetramyristoyl diphosphatidylglycerol or tetramyristoyl cardiolipin (TMCL) was purchased from Avanti Polar Lipids Inc. (Birmingham, AL). Double deionized H2O with a resistivity of 18.2 MΩ cm or deuterium oxide (Cambridge Isotope Laboratories) were used to prepare lipid–water mixtures. Homogeneous lipid dispersions were prepared by cycling the samples at least four times between −20 and 80 °C. The lipid–water ratio was 1/3 (wt/wt).2.2. DSC
Thermal analysis data were obtained with a differential scanning calorimeter DSC821e equipped with the high-sensitivity sensor HSS7 (Mettler-Toledo Co., Switzerland). The scan rate was 0.5 °C min−1.2.3. Synchrotron X-ray diffraction
SAXS and WAXS experiments were performed at the beamline BL16B1 of the Shanghai Synchrotron Radiation Facility (SSRF) (λ = 1.24 Å). The experimental setup is similar to that described in the ref. 17. A Rayonix SX-165 CCD detector was used to collect the X-ray data. A standard silver behenate sample was used for the calibration of diffraction spacings. X-ray scattering intensity patterns were recorded during 120 s exposure of the samples to the synchrotron beam. To acquire the SAXS data, we adjusted the sample-to-detector distance to 1805.0 mm, while to obtain the WAXS data, we fixed the sample-to-detector distance at 473.6 mm. A Linkam thermal stage (Linkam Scientific Instruments, the United Kingdom) was used for temperature control (±0.1 °C). The X-ray powder diffraction intensity data were analyzed using the program Fit2D.2.4. FTIR spectroscopy
FTIR spectra were recorded using a Nicolet 5700 Fourier transform infrared spectrometer with a DTGS detector in the range of 4000–900 cm−1 with a spectral resolution of 2 cm−1 and a zero filling factor of 2. Samples were coated onto the inner surfaces of a pair of CaF2 windows, which were mounted on a Linkam heating–cooling stage for temperature control (±0.1 °C). Spectra were recorded every ∼30 s and each spectrum consists of 16 scans. The peak fitting procedure of the IR band of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups was carried out using the PeakFit v4.05 software (AISN Software Inc.). The baseline was created by the two-point linear method and the peak type was Gaussian + Lorentz for all the peak fitting treatments.
O groups was carried out using the PeakFit v4.05 software (AISN Software Inc.). The baseline was created by the two-point linear method and the peak type was Gaussian + Lorentz for all the peak fitting treatments.
3. Results and discussion
3.1. DSC
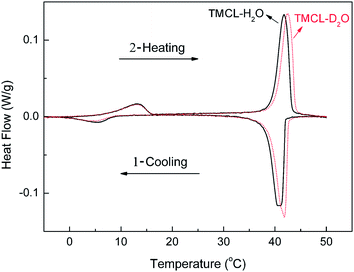
The thermotropic phase behaviors of TMCL–H2O (25 wt%) and TMCL–D2O (25 wt%) are shown in Fig. 2. For TMCL–H2O, upon cooling, a large, sharp exothermic peak at Tpeak = 40.8 °C appears, and further cooling to a temperature below 8.5 °C gives another exothermic peak. The following X-ray diffraction and FTIR data revealed that these two exothermic peaks correspond to fluid–gel transition and gel–subgel phase transition, respectively. Immediate reheating gives two endothermic peaks at Tpeak = 13.2 and 41.7 °C, corresponding to subgel–gel and gel–fluid phase transitions, respectively. The phase transition enthalpies of the two endothermic peaks are the same as the two corresponding exothermic peaks, and the values for the first and second endothermic peaks are 12.3 kJ mol−1 and 56.6 kJ mol−1. The thermotropic phase behavior of TMCL–D2O is almost the same as TMCL–H2O, except the slight upward shift (∼1 °C) of the second endothermic peak in TMCL–D2O. The nearly identical endothermic peaks at Tpeak = 13.2 °C for TMCL–H2O and TMCL–D2O indicate that the molecular rearrangements during subgel–gel transition is not significantly affect by the solvent. While for the second endothermic peak, the shift in peak position suggests that the molecular rearrangements in this gel–fluid transition involves the partition of water. The results are very similar with our previously reported results for DPPC–H2O and DPPC–D2O, where the subgel–gel transition also remained unchanged in the two different water environments, while the gel–fluid transition temperature was slightly higher in D2O.18To investigate the formation condition and kinetics of the crystalline phase, samples were incubated in the refrigerator (∼2 °C) for various time periods. We can see from Fig. 3 that incubation for 11 h results in the upward shift of the first endothermic peak from ∼13 °C to ∼15 °C, indicating that the lipid tail region becomes slightly more ordered during the low temperature incubation. After incubation for 24 h, this singular peak splits into two peaks at 17 and 19 °C, and further increasing the incubation time to 44 h, two peaks at 20 and 24 °C are seen. The two-peak pattern reached a constant shape and position at incubation times longer than 14 days, at 25 and 27 °C. These results show that the formation of the crystalline phase of TMCL is a very complex and slow process, and the subgel phase serves as a precursor for the formation of crystalline phase.
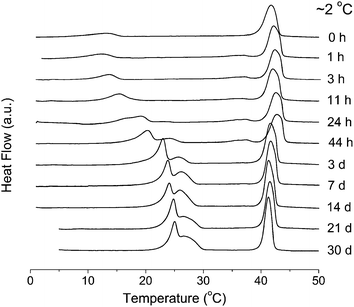 | ||
| Fig. 3 DSC heating results of TMCL–H2O (25 wt%) after incubations at ∼2 °C for different periods of time. | ||
The thermotropic phase behavior of TMCL in buffer solutions (Tris buffer and sodium phosphate buffer) has been investigated by Lewis et al., who found a “lamellar subgel (crystalline) to gel” transition (25–30 °C) and a “gel to lamellar liquid-crystalline (fluid)” (38–42 °C) transition upon heating the samples that had been fully equilibrated at low temperatures. They have also observed another endothermic peak at 14–18 °C when TMCL aqueous dispersions were reheated within a few hours after cooling to temperatures near 0 °C. However, the formation kinetics of the crystalline phase upon low temperature equilibration has not been uncovered. Besides, they have not assigned the endothermic peak at 14–18 °C, which actually corresponds to the “subgel–gel” transition observed at ∼13 °C in the present TMCL–water system. Furthermore, the occurrence of the peak at 14–18 °C observed by Lewis et al. was always together with the other two endothermic peaks (25–30 °C and 38–42 °C), suggesting that the subgel phase was not pure. The subgel and crystalline phases coexisted in the TMCL dispersions and upon heating, three endothermic peaks corresponding to “subgel to gel”, “crystalline to gel” and “gel to fluid” transitions, respectively, were observed in the DSC curve. In this work, by cooling the sample to −10 °C and immediately reheating it, only two endothermic peaks at ∼13 and 42 °C appeared in the DSC heating curve, which means that only two phase transitions occurred during heating: “subgel to gel” and “gel to fluid”. Thus, upon cooling to −10 °C, a pure subgel phase was obtained, which enables us to carefully and clearly elucidate its structure using the following X-ray and FTIR techniques.
3.2. Synchrotron X-ray diffraction
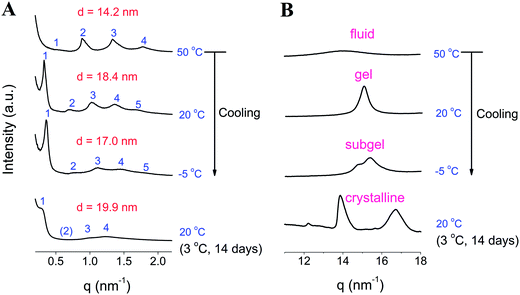
To study the phase states of the TMCL–water system, synchrotron X-ray diffraction studies were performed for the samples with the total lipid concentration of 25 wt%. Before measurement, the sample was divided into two portions for the SAXS and WAXS measurements. The SAXS data can reflect whether the phase state is multilameller and can determine the interlamellar spacing by the equation d = 2π/q. While peaks at the WAXS region can reflect the ordered tight packing of the lipid hydrocarbon tails. At 50 °C, as shown in Fig. 4A, the four scattering maxima vectors (qm) show a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, indicating that the sample is lamellar-structured. From the q values, we can obtain the repeat distances (the d values). The thus obtained average d value is 14.2 nm. From the WAXS data (Fig. 4B), we can see that the sample at 50 °C shows a broad peak at 14.0 nm−1 (d = 0.45 nm), which is a typical characteristic of fluid (liquid crystalline) phase.18,19 Cooling to 20 °C results in the formation of gel phase as evidenced by the appearance of a sharp WAXS peak centered at 15.1 nm−1 (d = 0.42 nm).18–20 The interlamellar spacing also shifts to 18.4 nm as calculated from the periodic SAXS peaks. Further cooling to −5 °C, the original singular WAXS peak splits into two peaks at 14.8 and 15.4 nm−1, indicating that the packing state in the lipid hydrocarbon region becomes more ordered in this phase state. Together with the FTIR results, we can see that this phase state has a packing state between gel and crystalline phase, which can be termed as “subgel” phase.18,21 Incubation at ∼3 °C for 14 days, the crystalline phase was obtained. The SAXS spectrum collected at 20 °C shows that the interlamellar spacing is 19.9 nm, and WAXS pattern changes significantly, with the occurrence of two main peaks at 13.9 and 16.7 nm−1, which was assigned to the (2 0) and (1 1) spacings of an orthorhombic subcell.16 The presence of these two peaks is a strong evidence of an ordered lipid tail packing.
4, indicating that the sample is lamellar-structured. From the q values, we can obtain the repeat distances (the d values). The thus obtained average d value is 14.2 nm. From the WAXS data (Fig. 4B), we can see that the sample at 50 °C shows a broad peak at 14.0 nm−1 (d = 0.45 nm), which is a typical characteristic of fluid (liquid crystalline) phase.18,19 Cooling to 20 °C results in the formation of gel phase as evidenced by the appearance of a sharp WAXS peak centered at 15.1 nm−1 (d = 0.42 nm).18–20 The interlamellar spacing also shifts to 18.4 nm as calculated from the periodic SAXS peaks. Further cooling to −5 °C, the original singular WAXS peak splits into two peaks at 14.8 and 15.4 nm−1, indicating that the packing state in the lipid hydrocarbon region becomes more ordered in this phase state. Together with the FTIR results, we can see that this phase state has a packing state between gel and crystalline phase, which can be termed as “subgel” phase.18,21 Incubation at ∼3 °C for 14 days, the crystalline phase was obtained. The SAXS spectrum collected at 20 °C shows that the interlamellar spacing is 19.9 nm, and WAXS pattern changes significantly, with the occurrence of two main peaks at 13.9 and 16.7 nm−1, which was assigned to the (2 0) and (1 1) spacings of an orthorhombic subcell.16 The presence of these two peaks is a strong evidence of an ordered lipid tail packing.
Temperature-dependent interlamellar spacing values (d) obtained by SAXS experiments (Fig. 5A) show that at temperatures below 8 °C, the d value starts to decrease, and it drops slightly from 18.3–18.4 nm (above 9 °C) to 17.0–17.1 nm (below 0 °C). The WAXS pattern in Fig. 5B shows that during the gel–subgel transition, the initial single sharp peak at 15.1–15.2 nm−1 starts to split into two peaks at 14.8 nm−1 (d = 0.424 nm) and 15.4 nm−1 (d = 0.408 nm) at temperatures below 8 °C. The change of a single WAXS peak to two peaks centered at d = 0.424 nm and 0.408 nm upon cooling from the gel phase is very similar to a previous work, where the authors found that cooling from gel phase results in the formation of a low-temperature ordered gel phase (a “subgel II (SGII)”-type phase).21 Such a formation of low-temperature ordered gel phases has been observed in several fully hydrated phosphatidylethanolamines (PEs) and phosphatidylcholines (PCs) with saturated chains as well as in dipalmitoylphosphatidylglycerol (DPPG).21 The split of the WAXS diffraction pattern from one reflection into two reflections suggests a transformation of the gel phase into a more ordered phase, with an orthorhombic chain packing (the Y-transition).21 The authors reported that such a Y-transition is fully reversible with heating–cooling treatments and displays in heating direction as a small endothermic event, in good agreement with our above DSC result (Fig. 2).
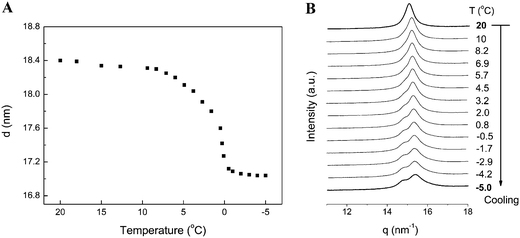 | ||
| Fig. 5 (A) Temperature-dependent d values obtained from SAXS curves of TMCL–H2O (25 wt%) during cooling from 20 °C to −5 °C, (B) temperature-dependent WAXS data during cooling from 20 °C to −5 °C. | ||
We have also investigated the dependence of the interlamellar spacing (d value) of TMCL–H2O system on water content. From the results shown in Fig. 6, we can see that the d value decreases with increasing TMCL content (or decreasing water content) for both the gel and fluid phases. This is not surprising for a charged lipid bilayer system.
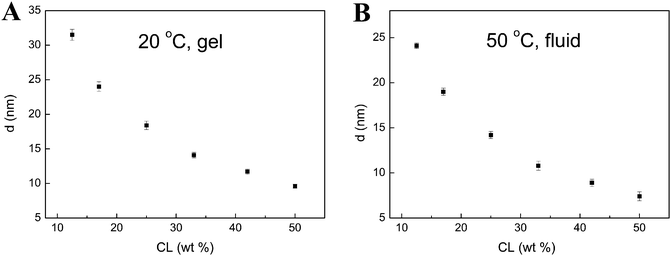 | ||
| Fig. 6 Dependencies of d value on TMCL content for the gel phase at 20 °C (A) and fluid phase at 50 °C (B) in TMCL–H2O system. | ||
From an intermolecular viewpoint, the interbilayer repulsive interactions include the electrostatic, hydration and undulation forces, while the interbilayer attractive interaction is the van der Waals force. The hydration force (mainly due to the difficulty to remove the structured water between lipid bilayers when two opposing membrane surfaces come close) assumes greater significance as the bilayers come into closer proximity.22,23 Besides, the undulation force (which is regarded to be the steric interactions caused by collision of bilayers) was also considered to take effect when the water layer is reduced.24,25 Considering that in our systems the bilayers are separated by a very large distance (with the interlamellar water layer thickness of 10–20 nm), the repulsive hydration and undulation forces are incidental. Thus, the most important repulsive force is the electrostatic interaction. The continuous swelling of the lipid repeat distance shows that even at the maximum water content that has been investigated in this work, the attractive force does not balance the repulsive. Besides, in comparison with the repeat distances (<10 nm) of TMCL in buffer solutions reported by Lewis et al.,16 the repeat distances obtained in water in the present work are much larger. This further suggests that the presence of salts can significantly decrease the thickness of the interlamellar water layer by the salt screening effect, which weakens the repulsive electrostatic forces between two neighboring lipid layers.
3.3. FTIR spectroscopy
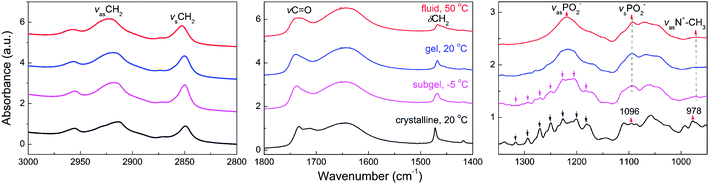
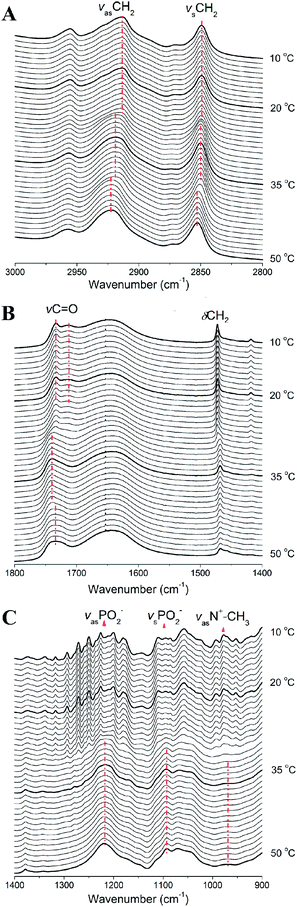
Having studied the general phase behavior and phase structure of TMCL by DSC and X-ray scattering, we will now turn to studying the submolecular details of the four phases using FTIR (see Fig. 7). By monitoring the characteristic IR bands of the polar headgroups (PO4− and N+–CH3), the interfacial group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and the apolar hydrocarbon chains (CH2), we can examine in detail the changes of the three different regions of the self-assembled phospholipid membranes during the phase transition processes. The 3000–2800 cm−1 region contains the CH2 asymmetric and symmetric stretching vibrations (denoted as νasCH2 and νsCH2, respectively), which reflect the conformational order of the lipid methylene chains. The peak position changes of the two stretching vibrations have been used frequently to follow the conformational order and the trans–gauche isomerization of the CH2 chains in lipid tail regions.26–33 The increase of wavenumber is partly due to the increase in the gauche conformers of methylene chains and partly due to the change in density or packing state of the chain region.34 At 50 °C in the fluid phase, νasCH2 and νsCH2 center at 2923 and 2853 cm−1, while they change to 2919 and 2850 cm−1 when cooled to 20 °C in gel phase, respectively, suggesting a decrease in the gauche conformers and a decrease in the density or packing order of the lipid tails during the fluid to gel phase transition. In the subgel phase, these two peaks change to 2917 and 2850 cm−1. The peak positions of the gel phase and subgel phase are very similar, indicating that the conformational order of the methylene tails of the two phase states are similar, with the all-trans conformers predominate in the two phase states. However, the 2850 cm−1 peak of the subgel phase is narrower than that in the gel phase, indicating the mobility of the methylene groups in the tail region of the subgel phase is further reduced as compared with that of the gel phase. In the crystalline phase, the two peaks shift to 2913 and 2849 cm−1, and the downward shift of the wavenumber suggests that the crystalline phase has the most abundant content of all-trans conformers and the lowest mobility in lipid tail region. The CH2 scissoring band (δCH2) is very sensitive to the intermolecular forces and can serve as a key band for examining the state of lateral packing of the methylene chains in various phases.26,28–30 The single sharp peak at 1472 cm−1 indicates that the methylene chains of the crystalline phase are packed in a triclinic 2D lattice. In gel state, this band shifts to 1468 cm−1, indicating the rearrangement of the methylene chain packing from triclinic to hexagonal. For a clearer comparison of the gel and subgel phases, enlarged figures containing the δCH2 and the C
O) and the apolar hydrocarbon chains (CH2), we can examine in detail the changes of the three different regions of the self-assembled phospholipid membranes during the phase transition processes. The 3000–2800 cm−1 region contains the CH2 asymmetric and symmetric stretching vibrations (denoted as νasCH2 and νsCH2, respectively), which reflect the conformational order of the lipid methylene chains. The peak position changes of the two stretching vibrations have been used frequently to follow the conformational order and the trans–gauche isomerization of the CH2 chains in lipid tail regions.26–33 The increase of wavenumber is partly due to the increase in the gauche conformers of methylene chains and partly due to the change in density or packing state of the chain region.34 At 50 °C in the fluid phase, νasCH2 and νsCH2 center at 2923 and 2853 cm−1, while they change to 2919 and 2850 cm−1 when cooled to 20 °C in gel phase, respectively, suggesting a decrease in the gauche conformers and a decrease in the density or packing order of the lipid tails during the fluid to gel phase transition. In the subgel phase, these two peaks change to 2917 and 2850 cm−1. The peak positions of the gel phase and subgel phase are very similar, indicating that the conformational order of the methylene tails of the two phase states are similar, with the all-trans conformers predominate in the two phase states. However, the 2850 cm−1 peak of the subgel phase is narrower than that in the gel phase, indicating the mobility of the methylene groups in the tail region of the subgel phase is further reduced as compared with that of the gel phase. In the crystalline phase, the two peaks shift to 2913 and 2849 cm−1, and the downward shift of the wavenumber suggests that the crystalline phase has the most abundant content of all-trans conformers and the lowest mobility in lipid tail region. The CH2 scissoring band (δCH2) is very sensitive to the intermolecular forces and can serve as a key band for examining the state of lateral packing of the methylene chains in various phases.26,28–30 The single sharp peak at 1472 cm−1 indicates that the methylene chains of the crystalline phase are packed in a triclinic 2D lattice. In gel state, this band shifts to 1468 cm−1, indicating the rearrangement of the methylene chain packing from triclinic to hexagonal. For a clearer comparison of the gel and subgel phases, enlarged figures containing the δCH2 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band (νC
O stretching band (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are shown in Fig. 8. The δCH2 band of the subgel phase contains two peaks at 1471 and 1468 cm−1, which has a lipid tail packing state different from the crystalline state (a single peak at 1472 cm−1) and the gel state (a single peak at 1468 cm−1). As has been revealed by our WAXS data, the two-peak pattern indicates an orthorhombic chain packing for the methylene groups. For the lipid headgroup vibrations (νasPO2−, νsPO2− and νasN+–CH3), they are sensitive to the change of hydrational state. The almost conserved band positions and band shapes of νasPO2− (at 1220 cm−1), νsPO2− (at 1094 cm−1) and νasN+–CH3 (at 972 cm−1) show that their hydrational states do not change during the fluid–gel transition. When cooling from gel to subgel phase, some peaks mainly assigned to the methylene wagging band progression occur in the 1360–1190 cm−1 region (marked with arrows in Fig. 7),35 and these peaks become more evident in the crystalline phase. The results show that the methylene groups in the tail region have the following packing order sequence: crystalline > subgel > gel, in accordance with the interpretation of δCH2. Since these small methylene wagging bands severely affect the original peak shape of the νasPO2− band, we cannot derive precise hydration information from this band. However, from the other two conserved bands of νsPO2− (at 1094 cm−1) and νasN+–CH3 (at 972 cm−1), we can see that the headgroup hydrational state is almost the same in the subgel phase as those in the gel and fluid phases. Upon converting to the crystalline phase, the νsPO2− and νasN+–CH3 shift to a higher wavenumber 1096 cm−1 and 978 cm−1, respectively, and the νasN+–CH3 band becomes much sharper. These results indicate that the dehydration occurs in the headgroups when the crystalline phase forms.
O) are shown in Fig. 8. The δCH2 band of the subgel phase contains two peaks at 1471 and 1468 cm−1, which has a lipid tail packing state different from the crystalline state (a single peak at 1472 cm−1) and the gel state (a single peak at 1468 cm−1). As has been revealed by our WAXS data, the two-peak pattern indicates an orthorhombic chain packing for the methylene groups. For the lipid headgroup vibrations (νasPO2−, νsPO2− and νasN+–CH3), they are sensitive to the change of hydrational state. The almost conserved band positions and band shapes of νasPO2− (at 1220 cm−1), νsPO2− (at 1094 cm−1) and νasN+–CH3 (at 972 cm−1) show that their hydrational states do not change during the fluid–gel transition. When cooling from gel to subgel phase, some peaks mainly assigned to the methylene wagging band progression occur in the 1360–1190 cm−1 region (marked with arrows in Fig. 7),35 and these peaks become more evident in the crystalline phase. The results show that the methylene groups in the tail region have the following packing order sequence: crystalline > subgel > gel, in accordance with the interpretation of δCH2. Since these small methylene wagging bands severely affect the original peak shape of the νasPO2− band, we cannot derive precise hydration information from this band. However, from the other two conserved bands of νsPO2− (at 1094 cm−1) and νasN+–CH3 (at 972 cm−1), we can see that the headgroup hydrational state is almost the same in the subgel phase as those in the gel and fluid phases. Upon converting to the crystalline phase, the νsPO2− and νasN+–CH3 shift to a higher wavenumber 1096 cm−1 and 978 cm−1, respectively, and the νasN+–CH3 band becomes much sharper. These results indicate that the dehydration occurs in the headgroups when the crystalline phase forms.
Fig. 9 illustrated the temperature-dependent FTIR spectra collected from heating the initial crystalline phase (10 °C) to the final fluid phase (50 °C). The results clearly show a “crystalline–gel” transition (at Tonset = 23 °C) and a subsequent “gel–fluid” phase transition (at Tonset = 40 °C) during heating, which well characterizes the phase states and molecular rearrangements of the corresponding DSC results (>14 days) shown in Fig. 3.
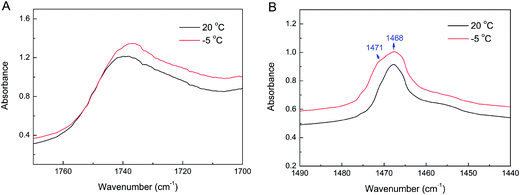
The band at 1765–1700 cm−1 is from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, which is partially overlapped by the absorbance band of H2O in the TMCL–H2O system (Fig. 7). To more precisely monitor the changes of the C
O stretching vibration, which is partially overlapped by the absorbance band of H2O in the TMCL–H2O system (Fig. 7). To more precisely monitor the changes of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorbance band, we used the TMCL–D2O system to avoid the absorbance of normal water centered at ∼1643 cm−1 in the TMCL–H2O system. The change of the C
O absorbance band, we used the TMCL–D2O system to avoid the absorbance of normal water centered at ∼1643 cm−1 in the TMCL–H2O system. The change of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band (νC
O stretching band (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) contour in the 1800–1650 cm−1 region for the TMCL–D2O system gives information on the alterations of the hydrational state and hydrogen bonding network in the lipid interface region, and the results are shown in Fig. 10. Peak fitting results reveal that for the fluid phase at 50 °C, the νC
O) contour in the 1800–1650 cm−1 region for the TMCL–D2O system gives information on the alterations of the hydrational state and hydrogen bonding network in the lipid interface region, and the results are shown in Fig. 10. Peak fitting results reveal that for the fluid phase at 50 °C, the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band consists of two component peaks at 1727.3 and 1743.0 cm−1, with the peak area percentages of 76.1% and 23.9%, respectively. In the gel phase at 20 °C, the band consists of two components at 1723.3 and 1741.3 cm−1, with the peak area percentages of 43.7% and 56.3%, respectively. At −5 °C in the subgel phase, the band consists of two components at 1720.9 and 1738.9 cm−1, with the peak area percentages of 47.1% and 52.9%, respectively. At 20 °C in the crystalline phase, the band consists of two components at 1716.9 and 1735.1 cm−1, with the peak area percentages of 46.6% and 53.4%, respectively. The components at 1720–1728 and 1738–1743 cm−1 may correspond to the hydrogen-bonded and non-hydrogen-bonded C
O band consists of two component peaks at 1727.3 and 1743.0 cm−1, with the peak area percentages of 76.1% and 23.9%, respectively. In the gel phase at 20 °C, the band consists of two components at 1723.3 and 1741.3 cm−1, with the peak area percentages of 43.7% and 56.3%, respectively. At −5 °C in the subgel phase, the band consists of two components at 1720.9 and 1738.9 cm−1, with the peak area percentages of 47.1% and 52.9%, respectively. At 20 °C in the crystalline phase, the band consists of two components at 1716.9 and 1735.1 cm−1, with the peak area percentages of 46.6% and 53.4%, respectively. The components at 1720–1728 and 1738–1743 cm−1 may correspond to the hydrogen-bonded and non-hydrogen-bonded C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species, respectively.36,37 This allows us to use the area percentage to estimate the hydrational state of the C
O species, respectively.36,37 This allows us to use the area percentage to estimate the hydrational state of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, and it is generally correct when evaluating the hydrational state of the gel and liquid crystalline phases.38 The results show that the fluid phase has a larger hydration degree than the other three phases (since the fluid phase has a much larger percentage of the 1720–1728 cm−1 band), and the gel and subgel phases have a similar hydrational state in the interfacial region. While for the crystalline phases, both the high and low wavenumber components experience a significant downward shift for 5–7 cm−1, and the band at 1735.1 cm−1 is much sharper than any of the other bands, which indicates that at least the packing state in the crystalline phase is different from the other three phases. The narrower band may indicate a reduced mobility in the interfacial region of the crystalline phase as compared with the other three phases.
O group, and it is generally correct when evaluating the hydrational state of the gel and liquid crystalline phases.38 The results show that the fluid phase has a larger hydration degree than the other three phases (since the fluid phase has a much larger percentage of the 1720–1728 cm−1 band), and the gel and subgel phases have a similar hydrational state in the interfacial region. While for the crystalline phases, both the high and low wavenumber components experience a significant downward shift for 5–7 cm−1, and the band at 1735.1 cm−1 is much sharper than any of the other bands, which indicates that at least the packing state in the crystalline phase is different from the other three phases. The narrower band may indicate a reduced mobility in the interfacial region of the crystalline phase as compared with the other three phases.
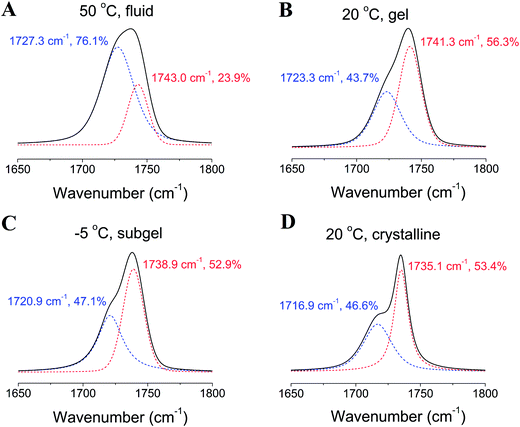 | ||
Fig. 10 Peak fitting results of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O region in the FTIR absorbance spectra of the four different phases in TMCL–D2O (25 wt%). O region in the FTIR absorbance spectra of the four different phases in TMCL–D2O (25 wt%). | ||
To sum up, the fluid-state TMCL molecules have well hydrated polar groups in the head and interface regions and loosely packed lipid tails. The gel and subgel phases have a similar headgroup and interface hydration/packing state as compared with the fluid phase, but they differs largely in the hydrophobic tail packing (both the gel and subgel phases have orderly packed tails, with the gel phase a hexagonal packing and the subgel phase an orthorhombic chain packing). While the crystalline phase has a very different lipid tail packing (triclinic) and a different packing/hydration in headgroup/interface as compared with the other three phases.
The transformation between the four phases of TMCL was illustrated in Fig. 11. The fluid–gel and gel–subgel transformations are reversible upon heating–cooling, while the formation of the crystalline phase is a slow process that needs a long-time incubation (typically > 14 days) at low temperatures (0–5 °C). The crystalline phase is stable at temperatures below 23 °C, and upon heating to above this temperature, it concerts to the gel phase. The phase transformation process involving the crystalline phase is not reversible. The subgel phase serves as a precursor for the formation of the crystalline phase. The present work gives a first detailed characterization of the phase state and phase transformation of this novel type of lipid bearing four acyl tails. The work will provide the fundamental for various other applications involving TMCL.
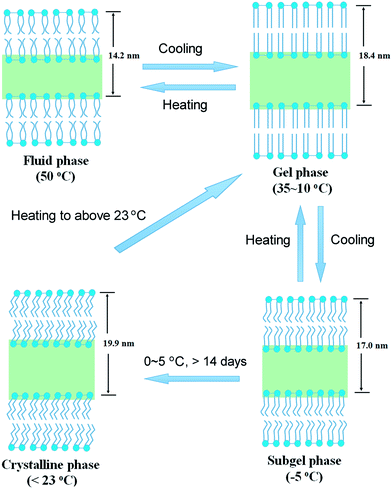 | ||
| Fig. 11 Schematic drawings showing the transformation of the four different phases of TMCL in water. | ||
4. Conclusions
In this work, we investigated the phase behavior of a four-tailed phospholipid, cardiolipin, in water. Four phase states have been characterized: fluid, gel, subgel and crystalline. The thermotropic phase transformations and the detailed phase structures and molecular conformations/packing states were studied by employing DSC, SAXS/WAXS and FTIR techniques. All the four phases are lamellar structured and the interlamellar spacings of the gel and fluid phases swell as the water content increases in pure water. The fluid to gel transition involves changes in the tail conformation (gauche to trans transition) and packing (loosely packed to ordered packed) and a dehydration process in the polar interface region, while the polar headgroups do not have significant hydrational/packing changes. The gel to subgel transition mainly involves the reorganization of the tail packing state (from hexagonal to orthorhombic), and this phase transition temperature does not differ in H2O or D2O media. Changing from subgel to crystalline phase needs a long incubation time at relatively low temperatures (0–5 °C), and this crystalline phase converts to gel phase upon heating. The transformations between fluid–gel and gel–subgel are reversible, while the phase transformation processes involving the crystalline phase are irreversible. The formation of the crystalline phase of TMCL is a very complicated and slow process, and the subgel phase serves as a precursor for the formation of crystalline phase. The present work will help researchers to use TMCL as a powerful building block for various constructions of self-assembly systems. It also provides helpful clues for researchers to use TMCL as a component to fabricate mimetic model cell membranes.Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (NSFC: Grant no. 21273130 and 21303017) and the Natural Science Foundation of Jiangsu Province (KB20130601). The SAXS and WAXS data were collected at the beamline BL16B1 of the Shanghai Synchrotron Radiation Facility (SSRF) with the assistances of the station scientists.References
- T. H. Haines and N. A. Dencher, FEBS Lett., 2002, 528, 35 CrossRef CAS.
- J. B. McMillin and W. Dowhan, Biochim. Biophys. Acta, 2002, 1585, 97 CrossRef CAS.
- M. Schlame, S. Brody and K. Y. Hostetler, Eur. J. Biochem., 1993, 212, 727 CrossRef CAS PubMed.
- J. B. McMillin and W. Dowhan, Biochim. Biophys. Acta, 2002, 1585, 97 CrossRef CAS.
- R. H. Houtkooper and F. M. Vaz, Cell. Mol. Life Sci., 2008, 65, 2493 CrossRef CAS PubMed.
- Y. P. Ow, D. R. Green, Z. Hao and T. W. Mak, Nat. Rev. Mol. Cell Biol., 2008, 9, 532 CrossRef CAS PubMed.
- V. E. Kagan, V. A. Tyurin, J. F. Jiang, Y. Y. Tyurina, V. B. Ritov, A. A. Amoscato, A. N. Osipov, N. A. Belikova, A. A. Kapralov, V. Kini, I. I. Vlasova, Q. Zhao, M. M. Zou, P. Di, D. A. Svistunenko, I. V. Kurnikov and G. G. Borisenko, Nat. Chem. Biol., 2005, 1, 223 CrossRef CAS PubMed.
- S. L. Iverson and S. Orrenius, Arch. Biochem. Biophys., 2004, 423, 37 CrossRef CAS PubMed.
- Y. N. Hong, J. Muenzner, S. K. Grimm and E. V. Pletneva, J. Am. Chem. Soc., 2012, 134, 18713 CrossRef CAS PubMed.
- J. Hanske, J. R. Toffey, A. M. Morenz, A. J. Bonilla, K. H. Schiavoni and E. V. Pletneva, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 125 CrossRef CAS PubMed.
- S. Pöyry, O. Cramariuc, P. A. Postila, K. Kaszuba, M. Sarewicz, A. Osyczka, I. Vattulainen and T. Róg, Biochim. Biophys. Acta, 2013, 1827, 769 CrossRef PubMed.
- C. W. T. Leung, Y. N. Hong, J. Hanske, E. G. Zhao, S. J. Chen, E. V. Pletneva and B. Z. Tang, Anal. Chem., 2014, 86, 1263 CrossRef CAS PubMed.
- A. Som, L. H. Yang, G. C. L. Wong and G. N. Tew, J. Am. Chem. Soc., 2009, 131, 15102 CrossRef CAS PubMed.
- R. N. A. H. Lewis and R. N. McElhaney, Biochim. Biophys. Acta, 2009, 1788, 2069 CrossRef CAS PubMed.
- A. Ortiz, J. A. Killian, A. J. Verkleij and J. Wilschut, Biophys. J., 1999, 77, 2003 CrossRef CAS.
- R. N. A. H. Lewis, D. Zweytick, G. Pabst, K. Lohner and R. N. McElhaney, Biophys. J., 2007, 92, 3166 CrossRef CAS PubMed.
- Z. H. Li, Z. H. Wu, G. Mo, X. Q. Xing and P. Liu, Instrum. Sci. Technol., 2014, 42, 128 CrossRef CAS.
- F. G. Wu, Q. Jia, R. G. Wu and Z. W. Yu, J. Phys. Chem. B, 2011, 115, 8559 CrossRef CAS PubMed.
- F. G. Wu, R. G. Wu, H. Y. Sun, Y. Z. Zheng and Z. W. Yu, Phys. Chem. Chem. Phys., 2014, 16, 15307 RSC.
- F. G. Wu, N. N. Wang, L. F. Tao and Z. W. Yu, J. Phys. Chem. B, 2010, 114, 12685 CrossRef CAS PubMed.
- B. Tenchov, R. Koynova and G. Rapp, Biophys. J., 2001, 80, 1873 CrossRef CAS.
- T. J. McIntosh and S. A. Simon, Annu. Rev. Biophys. Biomol. Struct., 1994, 23, 27 CrossRef CAS PubMed.
- M. L. Berkowitz, D. L. Bostick and S. Pandit, Chem. Rev., 2006, 106, 1527 CrossRef CAS PubMed.
- H. I. Petrache, N. Gouliaev, S. Tristram-Nagle, R. T. Zhang, R. M. Suter and J. F. Nagle, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1998, 57, 7014 CrossRef CAS.
- W. Z. Helfrich, Naturforscher, 1978, 33a, 305 CAS.
- R. N. A. H. Lewis and R. N. McElhaney, in Methods in Molecular Biology, ed. A. M. Dopico, Humana Press, Totowa, NJ, 2007, vol. 400, p. 207 Search PubMed.
- F. G. Wu, L. Chen and Z. W. Yu, J. Phys. Chem. B, 2009, 113, 869 CrossRef CAS PubMed.
- F. G. Wu, N. N. Wang and Z. W. Yu, Langmuir, 2009, 25, 13394 CrossRef CAS PubMed.
- F. G. Wu, J. J. Luo and Z. W. Yu, Langmuir, 2010, 26, 12777 CrossRef CAS PubMed.
- F. G. Wu, N. N. Wang, J. S. Yu, J. J. Luo and Z. W. Yu, J. Phys. Chem. B, 2010, 114, 2158 CrossRef CAS PubMed.
- F. G. Wu, J. S. Yu, S. F. Sun and Z. W. Yu, Langmuir, 2011, 27, 14740 CrossRef CAS PubMed.
- F. G. Wu, N. N. Wang, Q. G. Zhang, S. F. Sun and Z. W. Yu, J. Colloid Interface Sci., 2012, 374, 197 CrossRef CAS PubMed.
- F. G. Wu, J. S. Yu, S. F. Sun, H. Y. Sun, J. J. Luo and Z. W. Yu, Langmuir, 2012, 28, 7350 CrossRef CAS PubMed.
- J. Umemura, D. G. Cameron and H. H. Mantsch, Biochim. Biophys. Acta, 1980, 602, 32 CrossRef CAS.
- D. G. Cameron and H. H. Mantsch, Biophys. J., 1982, 38, 175 CrossRef CAS.
- A. Blume, W. Hubner and G. Messner, Biochemistry, 1988, 27, 8239 CrossRef CAS.
- A. Pérez-Lara, A. Ausili, F. J. Aranda, A. de Godos, A. Torrecillas, S. Corbalán-García and J. C. Gómez-Fernández, J. Phys. Chem. B, 2010, 114, 9778 CrossRef PubMed.
- R. N. A. H. Lewis, R. N. McElhaney, W. Pohle and H. H. Mantsch, Biophys. J., 1994, 67, 2367 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |