Direct synthesis of α-hydroxyacetophenones through molecular iodine activation of carbon–carbon double bonds†
Xia Wu,
Qinghe Gao,
Mi Lian,
Shan Liu and
Anxin Wu*
Key Laboratory of Pesticide & Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, P. R. China. E-mail: chwuax@mail.ccnu.edu.cn; Fax: +86 027-67867773; Tel: +86 027-67867773
First published on 7th October 2014
Abstract
An I2-promoted activation of carbon–carbon double bonds has been demonstrated. This simple and efficient domino process was utilized for the direct construction of α-hydroxyacetophenones in moderate to good yields from easily available styrenes at room temperature. This approach involved molecular iodine activation of carbon–carbon double bonds to form an iodonium intermediate. Moreover, TBHP was used as the sole effective oxidant and water was used as nucleophilic reagent in this reaction.
The α-hydroxy carbonyl moiety is an important structural unit that is widely found in drug molecules,1 natural products2 and as a potentially valuable synthetic intermediate in organic and medicinal chemistry.3 Due to this, there have been many reports of synthetic methodologies for the construction of α-hydroxy carbonyl compounds. Significantly, a dinuclear Pd(II) complex catalyzed chemo- and regioselective α-hydroxylation reaction of carbonyl compounds to build α-hydroxycarbonyl compounds by oxygen transfer from O2 has been reported by Ritter (Scheme 1a).4 Moreover, direct hydroxymethylation of aldehydes to construct α-hydroxycarbonyl compounds via NHC-catalyzed coupling of aldehydes with paraformaldehyde have been developed by Glorius (Scheme 1b).5 Very recently, Jiao and co-workers disclosed highly efficient and highly selective for tertiary C(sp3)–H bond cleavage to give α-hydroxycarbonyl compounds in the presence of Cs2CO3 and P(OEt)3 (Scheme 1c).6 However, in these transformations, few methods have been developed for direct oxidation of olefins approach to α-hydroxy ketones,7 due to the difficulty in introducing two oxygen atoms concurrently and oxidizing selectively. Moreover, to the best of our knowledge, no reports have been demonstrated by using styrenes to construct α-hydroxyacetophenones skeleton. In this context, the discovery and development of an I2-promoted direct activation and oxidation of styrenes to α-hydroxyacetophenones is highly desirable.
Molecule iodine as an old reagent has been widely used in organic synthesis for various organic functional group conversions due to its various functionalizing abilities and low cost, nontoxicity and easy availability.8 Over the past few years, iodine-catalysed reactions have been increasingly explored.9 In particular, the molecule iodine activation of carbon–carbon double bonds has received widespread interest in recent years.8c,10 In general, initial activation of the π-bond can be achieved either through a charge transfer complex or via an iodonium/iodonium intermediate.8c Subsequently, it could be attacked by a intermolecular nucleophile or intramolecular nucleophile to yield the respective target compounds.11–13 However, a method for the I2-promoted direct activation and oxidation of styrenes to α-hydroxyacetophenones has not been reported as yet. In this paper, an iodine-promoted activation of carbon–carbon double bonds is depicted to synthesize α-hydroxyacetophenones (Scheme 1d).
To initiate our study, we first tested the reaction of styrene in DMSO, TBHP at 55 °C. It was found that the reaction led to the desired product α-hydroxyacetophenone (2a) in a low yield of 21% (Table 1, entry 1). We found that, when the TBHP was changed from 0.6 equiv. to 4.0 equiv., the yield increased to 72% (Table 1, entries 2–7). Other temperature was also tested, which demonstrated that room temperature was the best choice (Table 1, entries 14–17). In addition, the reaction could not perform smoothly without I2 or TBHP (Table 1, entries 8 and 9). Various solvents, such as dimethyl formamide, acetonitrile, methanol, tertiary butanol and water were also examined, only the DMSO can perform this reaction smoothly (Table 1, entries 18–24). The use of aqueous hydrogen peroxide solution in the place of TBHP could not provide product 2a (Table 1, entry 25). After several experimental optimizations, we found that 1a (1.0 mmol) reacted with I2 (1.0 mmol), TBHP (4.0 mmol) in DMSO can afford the desired product in 80% yield (Table 1, entry 15) at room temperature.
| Entry | I2 (equiv.) | TBHP (equiv.) | Solvent | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), I2, TBHP, solvent (3 mL).b Isolated yields. | |||||
| 1 | 1.0 | TBHP (0.6) | DMSO | 55 | 21 |
| 2 | 1.0 | TBHP (1.2) | DMSO | 55 | 25 |
| 3 | 1.0 | TBHP (1.8) | DMSO | 55 | 43 |
| 4 | 1.0 | TBHP (2.6) | DMSO | 55 | 55 |
| 5 | 1.0 | TBHP (3.3) | DMSO | 55 | 59 |
| 6 | 1.0 | TBHP (4.0) | DMSO | 55 | 72 |
| 7 | 1.0 | TBHP (4.5) | DMSO | 55 | 70 |
| 8 | 1.0 | TBHP (0) | DMSO | 55 | 0 |
| 9 | 0 | TBHP (4.0) | DMSO | 55 | 0 |
| 10 | 0.5 | TBHP (4.0) | DMSO | 55 | 10 |
| 11 | 0.8 | TBHP (4.0) | DMSO | 55 | 12 |
| 12 | 1.2 | TBHP (4.0) | DMSO | 55 | 55 |
| 13 | 2.0 | TBHP (4.0) | DMSO | 55 | 46 |
| 14 | 1.0 | TBHP (4.0) | DMSO | r.t. | 80 |
| 15 | 1.0 | TBHP (4.0) | DMSO | 35 | 72 |
| 16 | 1.0 | TBHP (4.0) | DMSO | 45 | 71 |
| 17 | 1.0 | TBHP (4.0) | DMSO | 65 | 70 |
| 18 | 1.0 | TBHP (4.0) | DMF | r.t. | 0 |
| 19 | 1.0 | TBHP (4.0) | CH3CN | r.t. | 0 |
| 20 | 1.0 | TBHP (4.0) | H2O | r.t. | 0 |
| 21 | 1.0 | TBHP (4.0) | MeOH | r.t. | 0 |
| 22 | 1.0 | TBHP (4.0) | iPrOH | r.t. | 0 |
| 23 | 1.0 | TBHP (4.0) | tBuOH | r.t. | 0 |
| 24 | 1.0 | TBHP (4.0) | EtOH | r.t. | 0 |
| 25 | 1.0 | H2O2 (4.0) | DMSO | r.t | 0 |
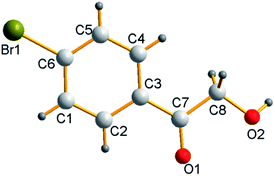
Under the optimal conditions, the scope of styrenes was next investigated. As shown in Scheme 2, a wide array of styrenes were examined in the reaction, with electron-neutral (4-H), electron-rich (3-CH3, 4-CH3) and electron-withdrawing (4-NO2, 4-CN) groups attached to the phenyl group could afford the corresponding products in moderate to good yields (60–83%, 2a–2e). Much to our satisfaction, functional groups attached to the styrenes such as fluoro, chloro, bromo (4-Cl, 4-Br, 4-F, 3-Br, 3-F, 2-F) were well tolerated under the optimized conditions (2f–2k, 62–73%), which could be used as an intermediate to synthesize more complex compounds. Trifluoromethyl groups on the phenyl rings of styrenes exhibited good reactivity (2l–2m, 62–67%). Furthermore, 2-vinylnaphthalene was also exhibited to proceeded smoothly to provide the desired products in good yield (2n, 72%). Moreover, the target compounds 2g was further determined by X-ray crystallographic analysis (Fig. 1).14
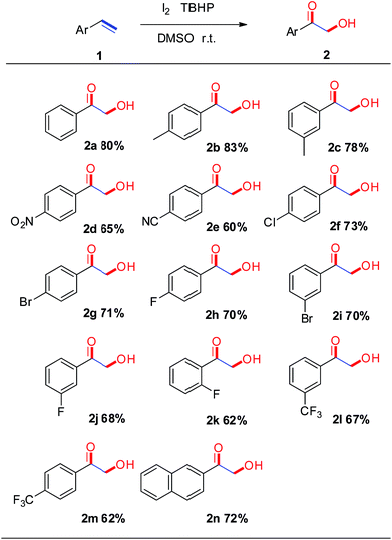 | ||
| Scheme 2 Synthesis of α-hydroxyacetophenones. (a) Reaction was performed with 1.0 mmol of 1, 1.0 mmol of I2, 4.0 mmol of TBHP in 3.0 mL of DMSO at room temperature. (b) Isolated yields provided. | ||
To gain some insight into the mechanism of the reaction, an O18-labeled H2O experiment with 1a using TBHP dissolution in decane was performed, and obvious detection of 18O–2aa by mass analysis suggested that the oxygen of hydroxy of 2a originated from H2O (Scheme 3).
According to the aforementioned information, we suggest a possible reaction mechanism which is depicted in Scheme 4. Initially, the iodine molecule promoted the transformation of 1-bromo-4-vinylbenzene 1g into the iodonium intermediate A, which was subsequently attacked by water to provide the intermediate B. Fortunately, we separated the by-product C which can give another abundant proof that intermediate A was attacked by water. Moreover, compound C was further determined by X-ray crystallographic analysis (Fig. 2).15 However, C could not be oxidized into final product 2g. Therefore, it is suggested that intermediate B was selectively oxidized to form the target product 2g.
In conclusion, a new method has been developed for the I2-promoted activation and oxidation of olefins. The protocol provides an efficient, green and readily accessible method to synthesize α-hydroxyacetophenones through oxidative 1,2-difunctionalization of styrenes under milder conditions.16 Further studies on the applications of this strategy will be reported in due course.
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (Grant 21032001, 21272085).Notes and references
-
(a) L. F. Fieser and M. Fieser in Steroids, Reinhold Publishing, NewYork, 1959, p. 600 Search PubMed
; (b) F. A. Davis and B. C. Chen, Chem. Rev., 1992, 92, 919 CrossRef CAS
; (c) A. E. Weber, M. G. Steiner, P. A. Krieter, A. E. Colletti, J. R. Tata, T. A. Halgren, R. G. Ball, J. J. Doyle and T. W. Schorn, J. Med. Chem., 1992, 35, 3755 CrossRef CAS
.
-
(a) F. C. Chen, C. F. Peng, I. L. Tsai and I. S. Chen, J. Nat. Prod., 2005, 68, 1318 CrossRef CAS PubMed
; (b) S. K. Wang, M. J. Huang and C. Y. Duh, J. Nat. Prod., 2006, 69, 1411 CrossRef CAS PubMed
; (c) R. Liu, Z. Lin, T. Zhu, Y. Fang, Q. Gu and W. Zhu, J. Nat. Prod., 2008, 71, 1127 CrossRef CAS PubMed
.
- For example, see:
(a) H. Liu, C. Dong, Z. G. Zhang, P. Y. Wu and X. F. Jiang, Angew. Chem., Int. Ed., 2012, 51, 12570 CrossRef CAS PubMed
; (b) T. Koike, K. Murata and T. Ikariya, Org. Lett., 2000, 2, 3833 CrossRef CAS PubMed
; (c) J. C. Lee, Y. S. Jin and J. H. Choi, Chem. Commun., 2001, 956 RSC
; (d) S. Matsunaga, N. Kumagai, S. Harada and M. Shibasaki, J. Am. Chem. Soc., 2003, 125, 4712 CrossRef CAS PubMed
; (e) B. M. Trost, J. Jaratjaroonphong and V. Reutrakul, J. Am. Chem. Soc., 2006, 128, 2778 CrossRef CAS PubMed
; (f) T. Ohkuma, N. Utsumi, M. Watanabe, N. Arai and K. Murata, Org. Lett., 2007, 9, 2565 CrossRef CAS PubMed
; (g) S. Baś, Ł. Woźniak, J. Cygan and J. Mlynarski, Eur. J. Org. Chem., 2013, 6917 CrossRef
.
- G. J. Chuang, W. Wang, E. Lee and T. Ritter, J. Am. Chem. Soc., 2011, 133, 1760 CrossRef CAS PubMed
.
- N. Kuhl and F. Glorius, Chem. Commun., 2011, 47, 573 RSC
.
- Y. F. Liang and N. Jiao, Angew. Chem., Int. Ed., 2014, 53, 548 CrossRef CAS PubMed
.
-
(a) B. B. Lohray, V. Bhushan and R. K. Kumar, J. Org. Chem., 1994, 59, 1375 CrossRef CAS
; (b) K. B. Sharpless and K. Akashi, J. Am. Chem. Soc., 1976, 98, 1986 CrossRef CAS
; (c) C. Bonini, L. Chiummiento, M. Funicello, P. Lupattelli and M. Pullez, Eur. J. Org. Chem., 2006, 80 CrossRef CAS
; (d) B. Plietker, J. Org. Chem., 2003, 68, 7123 CrossRef CAS PubMed
; (e) B. Plietker, Org. Lett., 2004, 6, 289 CrossRef CAS PubMed
; (f) Y. F. Zhang, Z. X. Shen, J. T. Tang, Y. Zhang, L. C. Kong and Y. W. Zhang, Org. Biomol. Chem., 2006, 4, 1478 RSC
.
-
(a) H. Togo and S. Iida, Synlett, 2006, 2159 CrossRef CAS PubMed
; (b) A. K. Banerjee, W. Vera, H. Mora, M. S. Laya, L. E. V. Bedoya and J. Cabrera, Sci. Ind. Res., 2006, 65, 299 CAS
; (c) P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, Chem.–Eur. J., 2012, 18, 5640 CrossRef PubMed
; (d) A. N. French, S. Bissmireb and T. Wirth, Chem. Soc. Rev., 2004, 33, 354 RSC
; (e) Y. M. Ren, C. Cai and R. C. Yang, RSC Adv., 2013, 3, 7182 RSC
; (f) T. J. Donohoe, M. A. Kabeshov, A. H. Rathia and E. D. Smithb Ian, Org. Biomol. Chem., 2012, 10, 1093 RSC
; (g) K. G. Ji, H. T. Zhu, F. Yang, X. Z. Shu, S. C. Zhao, X. Y. Liu, A. Shaukat and Y. M. Liang, Chem.–Eur. J., 2010, 16, 6151 CrossRef CAS PubMed
; (h) C. F. Wan, L. F. Gao, Q. Wang, J. T. Zhang and Z. Y. Wang, Org. Lett., 2010, 12, 3092 Search PubMed
.
-
(a) R. D. Richardson and T. Wirth, Angew. Chem., Int. Ed., 2006, 45, 4402 CrossRef CAS PubMed
; (b) T. Dohi and Y. Kita, Chem. Commun., 2009, 2073 RSC
.
- For selected review, see:
(a) A. K. Yadav, B. K. Singh, N. Singh and R. P. Tripathi, Tetrahedron Lett., 2007, 48, 6628 CrossRef CAS PubMed
; (b) M. J. Menezes, S. Manjrekar, V. Pai, R. E. Patre and S. G. Tilve, Indian J. Chem. Sect. B, 2009, 48, 1311 Search PubMed
; (c) K. C. Majumdar, K. Ray and S. Ponra, Tetrahedron Lett., 2010, 51, 5437 CrossRef CAS PubMed
; (d) A. Padwa and S. K. Bur, Tetrahedron, 2007, 63, 5341 CrossRef CAS PubMed
; (e) N. Shindoh, Y. Takemoto and K. Takasu, Chem.–Eur. J., 2009, 15, 12168 CrossRef CAS PubMed
.
- For reviews on nitrogen as nucleophile, see:
(a) F. Diaba, G. Puigbo and J. Bonjoch, Eur. J. Org. Chem., 2007, 3038 CrossRef CAS
; (b) W. Mai, S. A. Green, D. K. Bates and S. Fang, Synth. Commun., 2010, 40, 2571 CrossRef CAS
; (c) A. D. Jones and D. W. Knight, Chem. Commun., 1996, 915 RSC
.
- For reviews on oxygen as nucleophile, see:
(a) K. Kobayashi, K. Shikata, S. Fukamachi and H. Konishi, Heterocycles, 2008, 75, 599 CrossRef CAS
; (b) W. Gao, Y. Li, H. Zhang, M. Chang and K. Imafuku, J. Heterocycl. Chem., 2009, 46, 1107 CrossRef CAS
.
- For reviews on sulfur as nucleophile, see: T. Murai, H. Niwa, T. Kimura and F. Shibahara, Chem. Lett., 2004, 33, 508 CrossRef CAS
.
- ESI.†.
- ESI.†.
- C. Q. Chen, G. Z. Feng, G. Z. Zhang, Q. Zhao and G. S. Huang, Synlett, 2008, 3205 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 973925 and 973926. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra07012g |
| This journal is © The Royal Society of Chemistry 2014 |