A novel solution-controlled hydrogel coated mesh for oil/water separation based on monolayer electrostatic self-assembly†
Abstract
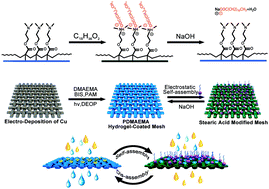
Solution-controlled hydrogel coated materials for oil/water separation have been successfully fabricated. The as-prepared mesh can switch between superhydrophilicity/underwater superoleophobicity and superhydrophobicity/superoleophilicity through self-assembly and dis-assembly pathways. The PDMAEMA hydrogel coated mesh is firstly prepared via photo induced free radical polymerization. A facile monolayer electrostatic self-assembly method was involved by immersing the hydrogel coated mesh in an ethanol solution of stearic acid for a few minutes. The stearic acid modified mesh converts to superhydrophobicity/superoleophilicity. More importantly, the mesh can return to the original state swiftly after the dis-assembly of the monolayer by immersion in a NaOH aqueous solution. The unique superiority of this material is that the transition of wettability can be achieved in situ, so that oil and water can pass through the mesh in sequence through the control of the NaOH solution. Therefore, the as-prepared meshes have great potential in practical applications dealing with both water-rich and oil-rich oil/water mixture separation with high separation efficiency (>99.3%). The synthetic method is simple, time saving and cost saving.

 Please wait while we load your content...
Please wait while we load your content...