Effect of biodegradable poly(ethylene adipate) with low molecular weight as an efficient plasticizer on the significantly enhanced crystallization rate and mechanical properties of poly(L-lactide)
Zhaobin Qiu* and
Pin Zhou
State Key Laboratory of Chemical Resource Engineering, Key Laboratory of Carbon Fiber and Functional Polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: qiuzb@mail.buct.edu.cn; Fax: +86-10-64413161
First published on 3rd October 2014
Abstract
The slow crystallization rate and low elongation at break are the main drawbacks of biodegradable poly(L-lactide) (PLLA) from a viewpoint of practical application. Such disadvantages of PLLA have been successfully resolved by blending with low molecular weight biodegradable poly(ethylene adipate) (PEA) in the present work. As a biodegradable plasticizer, PEA not only formed fully miscible and biodegradable polymer blends with PLLA but also apparently reduced the glass transition temperature of the blends. The increased chain mobility favored the nonisothermal cold and melt crystallization behaviors, increased the spherulitic growth rate, and accelerated the overall isothermal melt crystallization process of the blends; however, the blends had the same crystallization mechanism and crystal structures as neat PLLA. One of the exciting results was that the elongation at break value of an 80/20 PLLA/PEA blend was dramatically increased; therefore, for a wider practical application, the preparation of PLLA-based materials with fast crystallization rate, good mechanical properties, and complete biodegradability could be accomplished by the blending with a small amount of PEA with low molecular weight as an efficient plasticizer.
Introduction
Relative to other biodegradable polyesters, poly(L-lactide) (PLLA) has received more research interest, as the monomer for the preparation of PLLA can be obtained from renewable resources, thereby reducing the dependence on chemical resources.1–11 Because it is biodegradable and biocompatible, PLLA has found many end uses as both environmentally friendly materials and biomedical materials; however, the brittleness and slow crystallization rate are the main drawbacks of influencing its wider practical applications.9–14 In order to improve the ductility, the blending with ductile polymers is one of the most efficient and convenient ways of regulating the mechanical properties of PLLA.12–14 In previous work, we found that poly(ethylene succinate) (PES) may obviously improve the elongation at break of the PLLA/PES blends without losing the biodegradability.13Depending on the D-lactic acid unit, molecular weight, and crystallization conditions, PLLA does not crystallize or may only crystallize very slowly. Polymer crystallization consists of nucleation and crystal growth processes. To achieve a faster crystallization rate of PLLA, the addition of a nucleating agent is one of the most convenient choices. Till now, a number of nucleating agents have been found to be effective in accelerating the crystallization processes of PLLA-based materials under both nonisothermal and isothermal crystallization conditions. Some typical nucleating agents include polyglycolide, orotic acid, uracil, cyanuric acid, etc.15–19 In addition, some nanofillers are efficient nucleating agents for the PLLA-based nanocomposites, such as clay, polyhedral oligomeric silsesquioxanes, graphene, graphene oxide, multi-walled carbon nanotubes, and layered metal phosphonate.20–32 Many more nucleating sites may usually be provided in the presence of nucleating agent, thereby increasing the nucleation density of PLLA spherulites and accelerating the crystallization processes.15–32
In addition, the blending with a plasticizer is another effective way to promote the crystallization behavior of PLLA. Till now, some compounds or polymers may act as typical plasticizers for the plasticization of PLLA, such as triacetin, tributyl citrate, acetyl tri-n-butyl citrate, oligomeric lactic acid, triphenyl phosphate, poly(ethylene glycol), poly(propylene glycol), and poly(3-methyl-1,4-dioxan-2-one).33–39 In most cases, the presence of plasticizers can lower the glass transition temperature of PLLA-based materials; therefore, the increased chain mobility of the blends may enhance the crystallization behaviors of PLLA by mainly increasing the spherulitic growth rate and overall crystallization rate.33–39 It is interesting to note that the plasticizers can not only increase the ability of PLLA to crystallize under different crystallization conditions but also favor the plastic deformation of PLLA by significantly increasing the elongation at break and decreasing the elastic modulus as well as the yield strength.
The aim of this work is to study whether poly(ethylene adipate) (PEA) with low molecular weight may act as an efficient plasticizer of PLLA. In the present work, we prepared the PLLA/PEA blends with 10 and 20 wt% PEA to overcome the brittleness and slow crystallization rate of PLLA. The reasons why we chose low molecular weight PEA as a plasticizer are as follows. First, PEA has a low glass transition temperature value, which is comparable to that of poly(ethylene glycol), thereby making it a suitable candidate as a plasticizer. Second, PEA is miscible with PLLA, and no obvious phase separation occurs in the PLLA/PEA blends.40 Third, PEA is also biodegradable; therefore, the blending with PEA must still keep the biodegradability of PLLA. The research results are expected to be interesting and important from both polymer crystallization and practical application of PLLA-based materials viewpoints.
Experimental
PLLA (Mn = 9.56 × 104 g mol−1, Mw = 1.42 × 105 g mol−1) was kindly provided by Professor Yongjin Li of Hangzhou Normal University, China. PEA (Mn = 1.86 × 103 g mol−1, Mw = 2.69 × 103 g mol−1) was synthesized in our laboratory.41 The PEA sample used in this study had a glass transition temperature (Tg) of around −50 °C.Through a solution and casting process, the following three samples, including neat PLLA (100/0), 90/10, and 80/20 (weight ratio of PLLA to PEA) were prepared using chloroform as a solvent.
A TA Instruments differential scanning calorimeter (DSC) Q100 with a Thermal Analyst 2000 was used for the thermal analysis of neat PLLA and the PLLA/PEA blends under different conditions. For each sample, any previous thermal history must first be erased by annealing at 190 °C for 3 min. A heating rate of 20 °C min−1 was used to study the nonisothermal cold crystallization behaviors of the melt-quenched samples from the amorphous state, which had been reached by quenching to −80 °C at 60 °C min−1 after erasing any previous thermal history. A cooling rate of 5 °C min−1 was used to investigate the nonisothermal melt crystallization behavior, while a range of 125 to 132.5 °C was used to study the isothermal melt crystallization kinetics. The subsequent melting behaviors were studied at 20 °C min−1 after the isothermal melt crystallization processes at indicated crystallization temperature values for the determination of equilibrium melting point.
The spherulitic morphology and growth rates of neat and blended PLLA were studied with a polarized optical microscope (POM) (Olympus BX51) equipped with a temperature controller (Linkam THMS 600).
A Rigaku D/Max 2500 VB2+/PC X-ray diffractometer was used to study the crystal structures of neat and blended PLLA, which was operated 40 kV and 200 mA.
An Instron 1185 tensile testing machine was used to measure the mechanical properties of neat and blended PLLA at 20 mm min−1.
Results and discussion
Miscibility of PLLA/PEA blends
As shown in Fig. 1, the melt-quenched samples of neat PLLA and the PLLA/PEA blends were heated from the amorphous state at 20 °C min−1. Neat PLLA has a Tg of 62.5 °C. The PLLA/PEA blends only exhibit a single Tg below that of neat PLLA. The Tg values are significantly decreased to be 46.8 and 30.8 °C for the 90/10 and 80/20 samples, respectively. In brief, the blending with a small amount of PEA has obviously reduced the Tg values of the blends, indicating PLLA and PEA are miscible.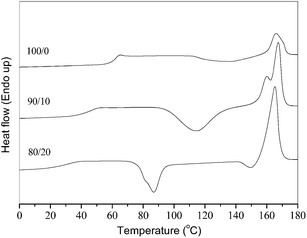 | ||
| Fig. 1 The melt-quenched samples of neat and blended PLLA were heated at 20 °C min−1 from the amorphous state. | ||
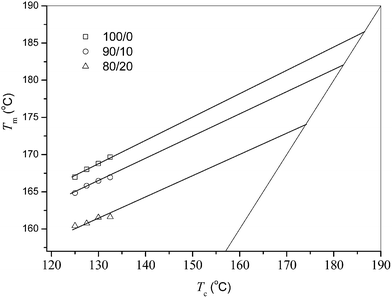
The depression of equilibrium melting point of PLLA/PEA blends can also confirm whether the two components are miscible or not.42 The equilibrium melting point (Tom) value is usually estimated by the Hoffmann–Weeks equation:
| Tm = ηTc + (1 − η)Tom | (1) |
Nonisothermal crystallization behavior studies of PLLA/PEA blends
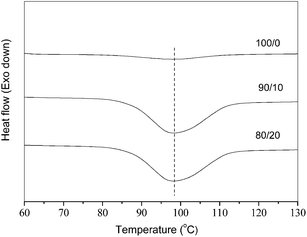
The effects of the blending with PEA on the nonisothermal crystallization behaviors of the blends were investigated with DSC. As shown in Fig. 1, the nonisothermal cold crystallization behaviors were investigated for the melt-quenched samples of neat and blended PLLA at 20 °C min−1. Neat PLLA has a cold crystallization peak temperature (Tch) of 136.1 °C with a cold crystallization enthalpy (ΔHch) of 12.4 J g−1. The 90/10 blend has a Tch of 114.8 °C with a ΔHch of 39.5 J g−1, while the 80/20 sample has a Tch of 86.9 °C with a ΔHch of 29.8 J g−1. From the aforementioned results, the blends show smaller Tch values than neat PLLA; moreover, the blends exhibit greater ΔHch values than neat PLLA. By using the heat of fusion of 93 J g−1 (100% crystalline PLLA),44 neat PLLA shows a crystallinity value of 13.3%. Considering the PLLA composition, the 90/10 and 80/20 samples show the normalized crystallinity values of 47.2% and 40.1%, respectively. In brief, the nonisothermal cold crystallization behaviors of PLLA have been apparently enhanced in the blends, which should arise from the obviously increased mobility of PLLA chains because of the smaller Tg values of the blends.Fig. 3 illustrates the DSC cooling traces of neat PLLA and its blends at 5 °C min−1. Both neat and blended PLLA crystallized, showing the broad crystallization exotherms. All the three samples show the nonisothermal melt crystallization peak temperature (Tp) values of around 98–99 °C, regardless of the PEA composition. However, the blending with PEA has significantly influenced the crystallization enthalpy (ΔHc) and crystallinity values. Neat PLLA shows a ΔHc value of only 6.0 J g−1; however, the 90/10 and 80/20 samples exhibit the ΔHc values of 32.7 and 33.7 J g−1, respectively. Considering the PLLA composition, the normalized crystallinity values are increased from 6.5% for neat PLLA to 39% and 45% for the 90/10 and 80/20 samples, respectively. The blending with PEA slightly affects the Tp values but significantly increases the ΔHc and crystallinity values of the blends; therefore, a small amount of PEA may obviously enhance the nonisothermal melt crystallization behavior of the blends. In brief, both the nonisothermal cold and melt crystallization behaviors of PLLA have been significantly enhanced after the blending with a small amount of PEA in their blends.
Overall isothermal melt crystallization kinetics of neat and blended PLLA
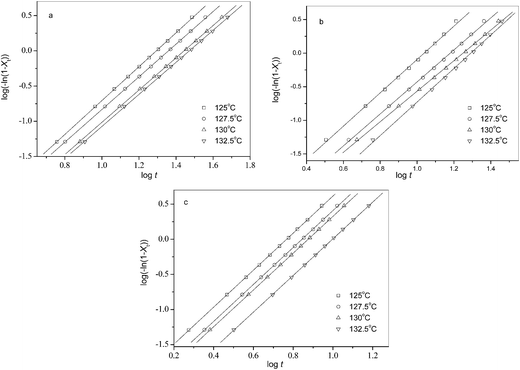
The isothermal melt crystallization kinetics of neat and blended PLLA was also studied at different Tc values. For all the three samples, the crystallization time became longer with increasing Tc. At the same Tc, the blends finished crystallization within shorter time than neat PLLA; moreover, the crystallization time of the 80/20 blend was shorter than that of the 90/10 sample, indicating that the former crystallized faster than the latter.The Avrami equation was applied to analyze the crystallization processes of neat and blended PLLA. The relative crystallinity (Xt) develops with crystallization time (t) as follows:
| 1 − Xt = exp(−ktn) | (2) |
The n and k values were calculated from Fig. 4. For comparison, Table 1 lists all the related parameters for all the three samples crystallized at different Tc values. Regardless of Tc and the PEA composition, the n values vary slightly between 2.3 and 2.6, suggesting the samples may all crystallize according to a three-dimensional truncated sphere growth with athermal nucleation mechanism.47 For each of the three samples, the k values increase with decreasing Tc; therefore, an acceleration of crystallization rate may occur at lower Tc because of larger supercooling when they have the same n values. To make a comparative study of the crystallization rates of neat and blended PLLA, the crystallization half-life time (t0.5) values were determined as follows:
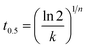 | (3) |
| Tc (°C) | 100/0 | 90/10 | 80/20 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | K (min−n) | t0.5 (min) | n | K (min−n) | t0.5 (min) | n | K (min−n) | t0.5 (min) | |
| 125 | 2.4 | 7.74 × 10−4 | 16.88 | 2.5 | 2.66 × 10−3 | 9.42 | 2.6 | 9.52 × 10−3 | 5.09 |
| 127.5 | 2.3 | 7.05 × 10−4 | 19.52 | 2.4 | 1.55 × 10−3 | 12.97 | 2.6 | 5.96 × 10−3 | 6.08 |
| 130 | 2.3 | 4.98 × 10−4 | 23.65 | 2.4 | 1.44 × 10−3 | 15.10 | 2.6 | 5.08 × 10−3 | 6.53 |
| 132.5 | 2.3 | 4.50 × 10−5 | 25.22 | 2.5 | 5.96 × 10−4 | 16.90 | 2.6 | 2.49 × 10−3 | 8.65 |
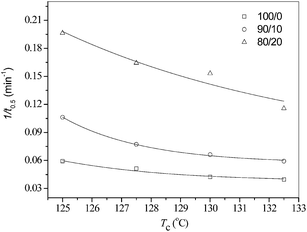
Table 1 also includes all the t0.5 values. The t0.5 values increase with an increase in Tc for each of the three samples, indicative of a slower crystallization rate at higher Tc. At the same Tc, the blends show smaller t0.5 values than neat PLLA; moreover, the 80/20 blend shows a smaller t0.5 value than the 90/10 sample. Accordingly, the reciprocal of t0.5 (1/t0.5) values were used to represent the isothermal melt crystallization rate.
Fig. 5 shows the Tc dependence of 1/t0.5 for neat and blended PLLA. Relative to neat PLLA, the blending with PEA has accelerated the isothermal melt crystallization process of PLLA in their blends at all Tc values. In conclusion, the blends have the same crystallization mechanism as neat PLLA; moreover, they crystallize faster than neat PLLA, after the blending with a small amount of PEA.
Spherulitic morphology and growth rates of neat and blended PLLA
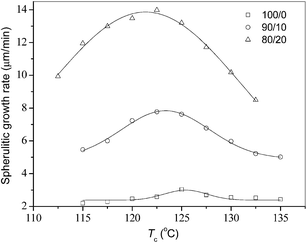
The spherulitic morphology and growth rates should also be studied for neat PLLA and the PLLA/PEA blends, because they may affect their final physical properties as well as biodegradation behaviors. Fig. 6 displays the POM images of neat and blended PLLA after crystallizing at 125 °C. Fig. 6a illustrates neat PLLA crystallized through a typical spherulitic growth with clear boundaries. The 90/10 and 80/20 samples present similar spherulitic morphologies as neat PLLA. Fig. 6b and c display that the whole spaces are filled with PLLA spherulites, suggesting that PEA must reside in the interlamellar and interfibrillar regions of PLLA spherulites.48,49Fig. 7 summarizes the measured spherulitic growth rates for all the three samples in a wide Tc range. Fig. 7 clearly shows that both neat and blended PLLA exhibit a bell-shape spherulitic growth rates within the investigated Tc range; moreover, with increasing the PEA composition the temperature values corresponding to maximum growth rates shift slightly downward to low temperature range. At the same Tc, neat PLLA shows slower spherulitic growth rate than blended PLLA; moreover, the spherulitic growth rate of an 80/20 sample is faster than that of a 90/10 sample. It is obvious that the blending with a small amount of PEA with low molecular weight favors faster spherulitic growth rates of the blends. The blending with PEA with low molecular weight significantly reduces the Tg values of the blends, especially at higher PEA content, and consequently increases the mobility of polymer chains of the blends; therefore, the ability to crystallize has been apparently enhanced for the blends, compared to neat PLLA.
Crystal structure study of neat and blended PLLA
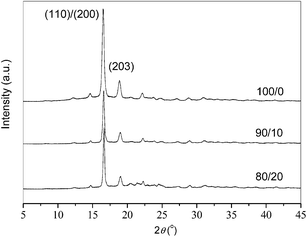
Fig. 8 illustrates the WAXD patterns of neat and blended PLLA, which were crystallized at 125 °C. When PLLA is crystallized from the melt, it may crystallize in order α modification at Tc > 120 °C or α′ modification at Tc < 100 °C, respectively; moreover, the formation of the former is thermodynamically preferential, while that of the latter is kinetically favored.50,51 In this research, neat and blended PLLA were crystallized at 125 °C; consequently, α form crystals must be developed for all the samples. Neat PLLA exhibits two main diffraction peaks at 16.51° and 18.87°, corresponding to (200)/(110) and (203) planes, respectively.50,51 The PLLA/PEA blends also present the similar WAXD patterns as that of neat PLLA, regardless of the PEA composition; therefore, the crystal structures of PLLA remain unmodified in the blends. In addition, neat PLLA shows a crystallinity value of around 50%, while the 90/10 and 80/20 samples show the crystallinity values of around 44% and 40%, respectively. In brief, the blends have the same crystal structures as neat PLLA; however, the blending with PEA slightly decreases the crystallinity values of the blends.Enhanced mechanical properties of PLLA/PEA blends
The influence of the blending with PEA on the mechanical properties of the PLLA/PEA blends was further studied. Fig. 9a displays the typical stress–strain curves of neat and blended PLLA, and Fig. 9b illustrates the enlarged stress–strain curves of all of the samples within the strain range of 0 and 15%. From Fig. 9, the tensile mechanical properties were determined.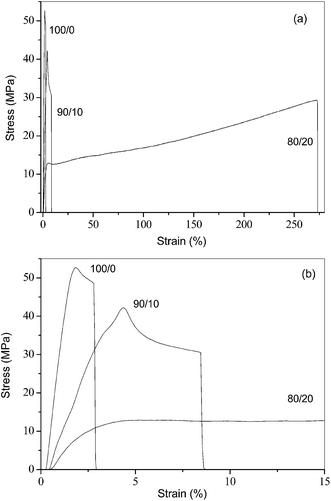 | ||
| Fig. 9 (a) Stress–strain curves of entire strain range and (b) magnification of 0–15% strain of neat and blended PLLA. | ||
Table 2 lists the tensile mechanical properties of the three samples. From Table 2, the blends show smaller Young's modulus and tensile strength values than neat PLLA; however, they exhibit greater elongation at break values than neat PLLA. The details are as follows.
| Samples | Young's modulus (MPa) | Tensile strength (MPa) | Elongation at break (%) |
|---|---|---|---|
| 100/0 | 2311.6 ± 129.0 | 52.9 ± 0.2 | 4.8 ± 0.1 |
| 90/10 | 1252.3 ± 32.6 | 42.4 ± 3.7 | 8.2 ± 1.2 |
| 80/20 | 474.3 ± 14.1 | 13.5 ± 0.9 | 284.4 ± 11.1 |
Neat PLLA has a Young's modulus of 2311.6 ± 129.0 MPa, a tensile strength of 52.9 ± 0.2 MPa, and an elongation at break of 4.8 ± 0.1%. For the PLLA/PEA blends, the Young's modulus values are reduced to be around 1252.3 ± 32.6 and 474.3 ± 14.1 MPa for the 90/10 and 80/20 samples, respectively; moreover, the tensile strength values are also decreased to be 52.9 ± 0.2 and 42.4 ± 3.7 MPa for the two samples with low and high PEA contents. One of the exciting results is that the elongation at break values have been significantly improved in the blends, relative to neat PLLA. For example, the elongation at break value is obviously increased to be around 8.2 ± 1.2% for a 90/10 blend from 4.8 ± 0.1% for neat PLLA; moreover, it is further tremendously improved to be around 284.4 ± 11.1% for an 80/20 sample. In other words, relative to that of neat PLLA, the elongation at break values have been increased by around 70% and 5800% for the 90/10 and 80/20 samples. Neat PLLA is brittle while the PLLA/PEA blends are very ductile, especially for the 80/20 blend; therefore, novel fully biodegradable PLLA-based materials with excellent ductile mechanical properties may be achieved through a simple polymer blending. On the basis of the chemical structures of PLLA and PEA, it is unlikely to show strong or weak chemical interactions between both of the components in their blended state. Therefore, the enhanced elongation at break should be attributed to the efficient plasticizer effect of PEA, which has actually lowered the glass transition temperature and improved the chain mobility of the blends.
Conclusions
In this work, the blending with a small amount of low molecular weight biodegradable PEA was used to overcome the disadvantages of slow crystallization rate and low elongation at break of PLLA, without losing the biodegradability. PEA was found to be miscible with PLLA, because the blends exhibited not only the single composition dependent Tg but also a depression of Tom. The chain mobility of the blends was increased by the obvious reduction of glass transition temperature values, thereby enhancing the crystallization behaviors of the blends under different crystallization conditions. First, the nonisothermal cold crystallization behaviors were obviously enhanced in the blends, as the cold crystallization peak temperature was decreased and the crystallinity values were increased. Second, the blending with PEA also favored the nonisothermal melt crystallization behaviors, as evidenced by the apparent increased crystallinity values, although the variation of nonisothermal melt crystallization peak temperatures was slight between neat PLLA and the PLLA/PEA blends. Third, the blends crystallized faster than neat PLLA during the overall isothermal melt crystallization process, as evidenced by the shorter crystallization half-time, indicating the blending with PEA also accelerated the overall isothermal melt crystallization processes of the blends. Fourth, the blending with PEA was also found to obviously enhance the growth rates of PLLA spherulites in the blends. The enhanced crystallization behaviors of the blends must be related to the increased chain mobility of PLLA, which was induced by the blending with PEA as an efficient plasticizer. After the blending with PEA, the blends show the same crystallization mechanism and crystal structures as neat PLLA. Moreover, the mechanical properties of the blends were also found to be improved, after the blending with a small amount of PEA. The elongation at break value of the 80/20 blend was dramatically increased to be around 284%, which was significantly than that of neat PLLA. Through a blending with a small amount of low molecular weight PEA, the wider practical application of PLLA-based materials would be achieved after overcoming their disadvantages of slow crystallization rates and unsatisfied mechanical properties.Acknowledgements
This research was supported by the National Natural Science Foundation, China (51221002 and 51373020).References and Notes
- M. Woodruff and D. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217 CrossRef CAS PubMed.
- J. Xu and B. Guo, Biotechnol. J., 2010, 5, 1149 CrossRef CAS PubMed.
- Z. Qiu, T. Ikehara and T. Nishi, Macromolecules, 2002, 35, 8251 CrossRef CAS.
- Y. Zhao and Z. Qiu, CrystEngComm, 2011, 13, 7129 RSC.
- G. Wang and Z. Qiu, Ind. Eng. Chem. Res., 2014, 53, 1712 CrossRef CAS.
- D. Aluthge, C. Xu, N. Othman, N. Noroozi, S. Hatzikiriakos and P. Mehrkhodavandi, Macromolecules, 2013, 46, 3965 CrossRef CAS.
- N. Othman, C. Xu, P. Mehrkhodavandi and S. Hatzikiriakos, Polymer, 2012, 53, 2443 CrossRef CAS PubMed.
- N. Othman, J. Ramírez, P. Mehrkhodavandi, J. Dorgan and S. Hatzikiriakos, J. Rheol., 2011, 55, 987 CrossRef CAS.
- S. Sinha Ray and M. Okamoto, Macromol. Rapid Commun., 2003, 24, 815 CrossRef.
- S. Saeidlou, M. Huneault, H. Li and C. Park, Prog. Polym. Sci., 2012, 37, 1657 CrossRef CAS PubMed.
- J. Raquez, Y. Habibi, M. Murariu and P. Dubois, Prog. Polym. Sci., 2013, 38, 1504 CrossRef CAS PubMed.
- L. Jiang, M. Wolcott and J. Zhang, Biomacromolecules, 2006, 7, 199 CrossRef CAS PubMed.
- J. Lu, Z. Qiu and W. Yang, Polymer, 2007, 48, 4196 CrossRef CAS PubMed.
- J. Zeng, Y. Li, Y. He, S. Li and Y. Wang, Ind. Eng. Chem. Res., 2011, 50, 6124 CrossRef CAS.
- H. Tsuji, K. Tashiro, L. Bouapao and J. Narita, Macromol. Mater. Eng., 2008, 293, 947 CrossRef CAS.
- Z. Qiu and Z. Li, Ind. Eng. Chem. Res., 2011, 50, 12299 CrossRef CAS.
- P. Pan, J. Yang, G. Shan, Y. Bao, Z. Weng and Y. Inoue, Macromol. Mater. Eng., 2012, 297, 670 CrossRef CAS.
- M. Weng and Z. Qiu, Thermochim. Acta, 2014, 577, 41 CrossRef CAS PubMed.
- H. Bai, W. Zhang, H. Deng, Q. Zhang and Q. Fu, Macromolecules, 2011, 44, 1233 CrossRef CAS.
- V. Krikorian and D. Pochan, Macromolecules, 2004, 37, 6480 CrossRef CAS.
- Z. Qiu and H. Pan, Compos. Sci. Technol., 2010, 70, 1089 CrossRef CAS PubMed.
- H. Pan and Z. Qiu, Macromolecules, 2010, 43, 1499 CrossRef CAS.
- J. Yu and Z. Qiu, ACS Appl. Mater. Interfaces, 2011, 3, 890 CAS.
- J. Yu and Z. Qiu, Thermochim. Acta, 2011, 519, 90 CrossRef CAS PubMed.
- J. Yu and Z. Qiu, Ind. Eng. Chem. Res., 2011, 50, 12579 CrossRef CAS.
- D. Wu, Y. Cheng, S. Feng, Z. Yao and M. Zhang, Ind. Eng. Chem. Res., 2013, 52, 6731 CrossRef CAS.
- H. Wang and Z. Qiu, Thermochim. Acta, 2011, 526, 229 CrossRef CAS PubMed.
- H. Wang and Z. Qiu, Thermochim. Acta, 2012, 527, 40 CrossRef CAS PubMed.
- Z. Qiu and W. Guan, RSC Adv., 2014, 4, 9463 RSC.
- Y. Zhao, Z. Qiu and W. Yang, Compos. Sci. Technol., 2009, 69, 627 CrossRef CAS PubMed.
- P. Pan, Z. Liang, A. Cao and Y. Inoue, ACS Appl. Mater. Interfaces, 2009, 1, 402 CAS.
- G. Papageorgiou, D. Achilias, S. Nanaki, T. Beslikas and D. Bikiaris, Thermochim. Acta, 2010, 511, 129 CrossRef CAS PubMed.
- N. Ljungberg and B. Wesslen, J. Appl. Polym. Sci., 2002, 86, 1227 CrossRef CAS.
- M. Baiardo, G. Frisoni, M. Scandola, M. Rimelen, D. Lips, K. Ruffieux and E. Wintermantel, J. Appl. Polym. Sci., 2003, 90, 1731 CrossRef CAS.
- O. Martin and L. Averous, Polymer, 2001, 42, 6209 CrossRef CAS.
- H. Xiao, W. Lu and J. Yeh, J. Appl. Polym. Sci., 2009, 113, 112 CrossRef CAS.
- K. Bechtold, M. Hillmyer and W. Tolman, Macromolecules, 2001, 34, 8641 CrossRef CAS.
- Z. Kulinski and E. Piorkowska, Polymer, 2005, 46, 10290 CrossRef CAS PubMed.
- Z. Kulinski, E. Piorkowska, K. Gadzinowska and M. Stasiak, Biomacromolecules, 2006, 7, 2128 CrossRef CAS PubMed.
- K. Okamoto, T. Ichikawa, T. Yokohara and M. Yamaguchi, Eur. Polym. J., 2009, 45, 2304 CrossRef CAS PubMed.
- H. Wu and Z. Qiu, Ind. Eng. Chem. Res., 2012, 51, 13323 CrossRef CAS.
- T. Nishi and T. Wang, Macromolecules, 1975, 8, 909 CrossRef CAS.
- J. Hoffman and J. Weeks, J. Chem. Phys., 1965, 42, 4301 CrossRef CAS PubMed.
- E. Fischer, H. Sterzel and G. Wegner, Kolloid Z. Z. Polym., 1973, 251, 980 CAS.
- M. Avrami, J. Chem. Phys., 1940, 8, 212 CrossRef CAS PubMed.
- M. Avrami, J. Chem. Phys., 1941, 9, 177 CrossRef CAS PubMed.
- B. Wunderlich, Macromolecular Physics, Academic Press, New York, 1976, vol. 2 Search PubMed.
- H. Keith and F. Padden Jr, J. Appl. Phys., 1964, 35, 1270 CrossRef CAS PubMed.
- H. Keith and F. Padden Jr, J. Appl. Phys., 1964, 35, 1286 CrossRef CAS PubMed.
- J. Zhang, Y. Duan, H. Sato, H. Tsuji, I. Noda, S. Yan and Y. Ozaki, Macromolecules, 2005, 38, 8012 CrossRef CAS.
- P. Pan and Y. Inoue, Prog. Polym. Sci., 2009, 34, 605 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |