Effect of edge modification on transport properties of finite-sized, graphene nanoribbon-based molecular devices
Abstract
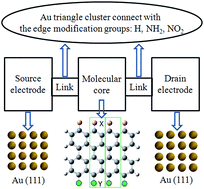
The transport mechanisms of several finite-sized, graphene nanoribbon-based junctions have been computationally investigated using density functional theory and Green's functional method. Acceptor-type and donor-type functional groups were introduced, serving as electrode connection and edge modification, respectively. The introduction of acceptor and donor groups improve the electron transmission probabilities by several orders of magnitude. The electronic coupling mode, carrier concentration and distribution, and molecular orbital can be manipulated by the modification groups.

 Please wait while we load your content...
Please wait while we load your content...