Rational synthesis of F-doped iron oxides on Al2O3(0001) single crystals
G. Carraroa,
A. Gasparotto*a,
C. Maccatoa,
E. Bontempib,
O. I. Lebedevc,
C. Sadad,
S. Turnere,
G. Van Tendelooe and
D. Barrecaf
aDepartment of Chemistry, Padova University and INSTM, 35131 Padova, Italy. E-mail: alberto.gasparotto@unipd.it
bChemistry for Technologies Laboratory, University of Brescia, 25123 Brescia, Italy
cLaboratoire CRISMAT, CNRS-ENSICAEN, UMR 6508, 14050 Caen Cedex 4, France
dDepartment of Physics and Astronomy, Padova University, 35131 Padova, Italy
eEMAT, Antwerp University, 2020 Antwerpen, Belgium
fIENI-CNR and INSTM, Department of Chemistry, Padova University, 35131 Padova, Italy
First published on 2nd October 2014
Abstract
A plasma enhanced-chemical vapor deposition (PE-CVD) route to Fe2O3-based materials on Al2O3(0001) single crystals at moderate growth temperatures (200–400 °C) is reported. The use of the fluorinated Fe(hfa)2TMEDA (hfa = 1,1,1,5,5,5-hexafluoro-2,4-pentanedionate; TMEDA = N,N,N′,N′-tetramethylethylenediamine) molecular precursor in Ar/O2 plasmas enabled an in situ F-doping of iron oxide matrices, with a fluorine content tunable as a function of the adopted preparative conditions. Variations of the thermal energy supply enabled control of the system phase composition, resulting in γ-Fe2O3 at 200 °C and α-Fe2O3 nanostructures at higher deposition temperatures. Notably, at 400 °C the formation of highly oriented α-Fe2O3 nanocolumns characterized by an epitaxial relation with the Al2O3(0001) substrate was observed. Beside fluorine content, phase composition and nano-organization, even the system optical properties and, in particular, energy gap values, could be tailored by proper modifications of processing parameters.
1. Introduction
In the last few decades, iron(III) oxides have acquired a prominent role in various technological fields. For instance, α-Fe2O3 (hematite) has emerged as an outstanding electrode material for Graetzel cells and as an efficient catalyst/photocatalyst in various important reactions.1–3 More recently, β- and ε-Fe2O3 have been reported to possess a catalytic activity even higher than hematite for solar hydrogen generation,4 whereas γ-Fe2O3 (maghemite) is regarded as a promising magnetic material in particular for its superparamagnetic behavior.1,5The extensive use of iron(III) oxides takes advantage not only of their non-toxicity, abundance and low cost, but also of the diversified properties of the various Fe2O3 polymorphs, providing such materials a strong potential for technological end-uses.1,6–8 The functional behavior of Fe2O3 can be further tailored via chemical modification, for instance by the introduction of metallic nanoparticles or anionic doping,9–11 the latter being much less explored. In particular, fluorine doping is the focus of an intense research interest, motivated by the possibility of tailoring iron oxide electrical, optical and chemical properties. For instance, F-doping can passivate defect states, enhance surface reactivity, and tune both electrical conductivity and light absorption properties.9,12–20 Accordingly, fluorine doping candidates itself as a powerful tool to improve the system behavior in several applications encompassing photocatalysis, optoelectronics, energy storage and gas sensing.8,15,18,19,21,22
In addition, iron oxide properties can be tailored and optimized through a careful control of its nano-organization. For instance, in view of eventual photocatalytic/photoelectrochemical utilizations, columnar nanostructures join a high surface area with the possibility of absorbing a significant light fraction, while providing short carrier transport distances to the electrolyte.9,23 Furthermore, the formation of heterojunctions at the interface with a single crystal substrate, or with a second nanostructured material, can suppress recombination processes, resulting in a higher quantum efficiency.3,21
On this basis, herein we report on the synthesis and chemico-physical characterization of F-doped iron oxides on Al2O3(0001) single crystals. As anticipated, fluorine doping and epitaxial growth play an important role from an applicative point of view, since they directly impact the material functional behavior. Nonetheless, fundamental understanding of structure/property interrelations, with particular regard to the role of doping and epitaxy on nucleation/growth phenomena, is still limited and represents a bottleneck for further research progresses. Specifically, F-doping of Fe2O3 or other metal oxides has been reported to influence crystallinity and, more specifically, to affect strain, grain size/shape and growth orientation.7,15,17,19,21,22,24 On the other hand, the use of single crystal substrates can not only stabilize a specific polymorph, but also impact interface quality and surface faceting.2,25–27
In this work, F-doped iron oxides are synthesized by PE-CVD on Al2O3(0001) substrates starting from the fluorinated Fe(hfa)2TMEDA molecular compound, that acts as single-source precursor for both Fe and F. Special attention was devoted to a detailed characterization of the system composition, morphology, nanostructure and optical properties by the complementary use of X-ray photoelectron spectroscopy (XPS), secondary ion mass spectrometry (SIMS), field emission-scanning electron microscopy (FE-SEM), bi-dimensional X-ray diffraction (XRD2), (high resolution)-transmission electron microscopy [(HR)-TEM], electron diffraction (ED), and optical absorption measurements. The most relevant data are presented and critically discussed as a function of preparative conditions.
2. Experimental section
2.1 Synthesis
The Fe(hfa)2TMEDA precursor was synthesized following a previously reported literature procedure.28 Al2O3(0001) single crystals (10 × 10 × 1 mm3, one-side polished) were purchased from Crystal GmbH (Berlin, Germany) and used as growth substrates without any further treatment. Deposition experiments were carried out using a two electrode radio frequency (RF; ν = 13.56 MHz) PE-CVD apparatus29 in Ar/O2 (gas flow rates = 15 and 20 sccm, respectively) plasmas. The total pressure, deposition time and RF power were kept constant at 1.0 mbar, 60 min and 10 W, respectively, using an interelectrode distance of 6.0 cm. Fe(hfa)2TMEDA was vaporized at 65 °C by means of an oil bath, and its vapors were transported into the reaction chamber by means of an Ar flow (rate = 60 sccm). The feeding gas lines were heated at 140 °C to prevent precursor condensation phenomena. Under the above processing conditions, experiments were carried out at 200, 300 and 400 °C to investigate the influence of growth temperature on the properties of the resulting iron oxide-based deposits.2.2 Characterization
XPS analyses were performed on a Perkin-Elmer Φ5600ci spectrometer, using a standard AlKα excitation source (1486.6 eV), at working pressures lower than 10−8 mbar. Binding energies (BEs, standard deviation = ±0.2 eV) were corrected for charging assigning a value of 284.8 eV to the adventitious C1s line.30 Ar+ sputtering was carried out at 3.0 kV (area = 2 × 2 mm2, Ar partial pressure = 5 × 10−8 mbar). Atomic percentages (at.%) were calculated by signal integration using standard PHI V5.4A sensitivity factors. Peak fitting was performed by a least-squares procedure, adopting Gaussian–Lorentzian peak shapes.SIMS analyses were carried out by means of an IMS 4f mass spectrometer (Cameca) using a 14.5 keV Cs+ primary beam (current = 25 nA, stability = 0.3%) and by negative secondary ion detection, adopting an electron gun for charge compensation. Beam blanking mode and high mass resolution configuration were adopted. Signals were recorded rastering over an area of 175 × 175 μm2 and detecting secondary ions from a sub-region close to 8 × 8 μm2 in order to avoid crater effects.
FE-SEM micrographs were collected by a Zeiss SUPRA 40VP instrument, with a primary beam voltage of 10 kV. The mean nanoaggregate size was evaluated through the SmartSEM® software by averaging over 20 independent measurements for each specimen.
XRD2 images were collected by a Dymax-RAPID X-ray diffractometer with a cylindrical imaging plate detector, that allows collecting diffraction data in the ranges 2θ = 0 to 160° (horizontally) and 2θ = −45 to +45° (vertically) upon using CuKα radiation. The incident beam collimators enable different spot sizes to be projected onto the sample. In this work, measurements were performed in reflection mode, adopting a collimator diameter of 300 μm and an exposure time of 30 min for each XRD2 pattern.
(HR)-TEM and ED experiments were carried out on a FEI Tecnai G2 30 UT microscope operated at 300 kV. High-angle annular dark-field STEM (HAADF-STEM) experiments were performed by an aberration-corrected Titan “cubed” microscope, operated at 300 kV. The used convergence semi-angle α and HAADF detector inner semi-angle β were 21 and 50 mrad, respectively. Specimens for cross-sectional (CS) and plane-view (PV) observations were prepared by mechanical polishing down to a thickness of approximately 20 μm, followed by Ar+ ion milling under grazing angle down to electron transparency.
Optical absorption spectra were recorded by means of a Cary 5E (Varian) dual-beam spectrophotometer with a spectral bandwidth of 1 nm, operating in transmission mode at normal incidence. For each spectrum, the substrate contribution was subtracted. Optical band-gaps were estimated from Tauc plots (αhν)2 vs. hν.5,18,31
3. Results and discussion
3.1 Composition
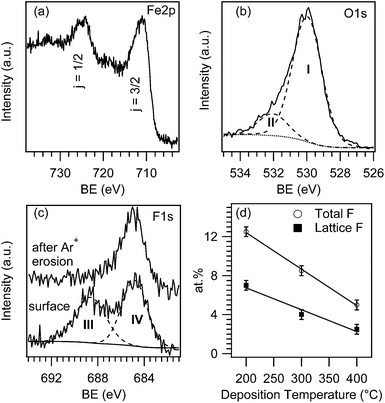
In order to investigate the system surface and in-depth composition, XPS analyses were preliminarily carried out. Irrespective of the synthesis conditions, all samples were characterized by the presence of iron, oxygen, fluorine and carbon, the latter being limited to the outermost deposit layers. Fig. 1a displays the Fe2p surface peak for an iron oxide sample grown at 300 °C. The Fe2p3/2 signal was located at a BE of 711.0 eV with a spin–orbit separation of 13.5 eV. These data, along with the low intensity of shake-up satellites, are in good agreement with the presence of iron(III) oxide free from other Fe-containing species.10,11,32–35 Accordingly, the main contribution to the O1s peak (see Fig. 1b, component I, 85.6% of the overall O signal) at 530.1 eV was attributed to lattice oxygen in Fe2O3.26,32,35,36 The second band (component II) located at BE = 531.9 eV could be ascribed to adsorbed –OH groups and carbonate species arising from atmospheric exposure.32,33As can be observed, the F1s surface peak could be decomposed by means of two bands (Fig. 1c). Whereas the high BE one (component III, BE = 688.5 eV) was due to CFx species arising from an incomplete precursor decomposition,10,33,37,38 component IV at 684.8 eV was traced back to F incorporation into iron oxide lattice,7,10,32,38 indicating the formation of F-doped Fe2O3. Interestingly, the former signal disappeared upon a mild sputtering, highlighting that the presence of CFx moieties was limited to the outermost layers. Conversely, lattice fluorine was still clearly detectable after Ar+ erosion (Fig. 1c). It is also worth noting that both the overall and lattice F surface content underwent a linear decrease upon increasing the deposition temperature (Fig. 1d), as already observed under similar conditions.9,39
In order to investigate the fluorine distribution in the inner material layers, XPS and SIMS depth profiling were carried out (Fig. 2). Fig. 2a shows a representative XPS depth profile. The slight decrease of oxygen at.% occurring after the first 15 min of erosion is likely due to preferential sputtering phenomena, responsible also for the apparent increase of iron content.30 Fluorine amount progressively decreased during the first erosion cycles and subsequently reached a constant value of ca. 2 at.% in the inner sample region. This behavior was related to the disappearance of surface CFx species upon erosion, resulting in the presence of the sole lattice fluorine, that was homogeneously distributed in the iron oxide matrix.
 | ||
| Fig. 2 (a) XPS depth profile for a sample deposited at 300 °C. SIMS profiles for specimens grown at (b) 200 °C, (c) 300 °C and (d) 400 °C. | ||
The results of SIMS analyses (Fig. 2b–d) agreed to a good extent with XPS ones. In particular, irrespective of the adopted growth temperature, Fe, O and F ionic yield profiles were almost parallel throughout the entire nanodeposit thickness, indicating an uniform chemical composition and an even doping level. In addition, all samples showed a sharp and well defined interface with the substrate, allowing an accurate calculation of the deposit thickness. The pertaining values were 200 ± 15, 260 ± 15, and 170 ± 15 nm at 200, 300 and 400 °C, respectively.
3.2 Morphology
The influence of processing conditions on the system morphology was analyzed by FE-SEM (Fig. 3). At 200 °C, plane-view investigation evidenced the formation of leaf-like lamellar nanostructures with an average lateral size and thickness of 80 and 25 nm, respectively. As evidenced by a closer micrograph inspection, such structures were composed by smaller and randomly oriented interconnected particles, suggesting the occurrence of a polycrystalline material. | ||
| Fig. 3 FE-SEM PV micrographs of the iron oxide materials. The inset shows a cross-sectional image of the 400 °C sample. | ||
Upon increasing the growth temperature to 300 °C, the deposit morphology was only partially reminiscent of the previous one. In fact, the observed lamellar structures exhibited a more pronounced faceting and, in some cases, a well evident rectangular prism habit, with an in-plane size of 120 nm × 70 nm.
At 400 °C, the system morphology underwent significant variations with respect to the previous cases. In fact, homogeneously distributed columnar structures aligned perpendicularly to the substrate surface could be observed. These nano-columns were characterized by faceted tips, with average diameter and length values of 30 and 170 nm, respectively. The obtainment of this nano-organization at the highest deposition temperature suggested a marked influence of the Al2O3(0001) substrate on Fe2O3 nucleation and growth, as discussed in detail below.
3.3 Structure
The microstructural properties of the Fe2O3 nanomaterials were analyzed by two-dimensional X-ray diffraction and the pertaining XRD2 maps are reported in Fig. 4, along with the corresponding integrated patterns. At the lowest deposition temperature (200 °C), signals at 2ϑ = 30.0, 35.6, 63.7 and 65.0° were detected, and attributed to the (220), (311), (441) and (530) reflections of γ-Fe2O3 (maghemite).40 On the other hand, the specimen deposited at 300 °C displayed peaks at 24.1, 33.0, 35.5, 40.7, 49.2, 53.8, 57.3, 62.2, 63.6 and 74.9°, attributable to the (012), (104), (110), (113), (024), (116), (018), (214), (300) and (220) reflections of α-Fe2O3 (hematite).41 In this case, a comparison with the powder reference spectrum indicated a preferred orientation along the 〈110〉 direction. The observed phase transition from maghemite (200 °C) to hematite (300 °C) was not surprising, and could be explained by the higher thermodynamic stability of α-Fe2O3.1,6,34 | ||
| Fig. 4 XRD2 maps and corresponding integrated spectra of specimens obtained at 200 and 300 °C. Reflections expected for γ-Fe2O3 (ref. 40) and α-Fe2O3 (ref. 41) are marked by continuous and dashed lines, respectively. | ||
Nevertheless, at 400 °C no reflections other than the substrate ones could be appreciated in the XRD2 pattern (not reported). A similar finding suggests the occurrence of an epitaxial/oriented growth strongly affected by the underlying Al2O3(0001) support since, in a similar case, a full overlap between the substrate and the deposit reflections is expected.42
In order to attain a deeper insight into this phenomenon, a detailed TEM analysis was carried out on the sample synthesized at 400 °C. Low-magnification and high resolution PV and CS TEM images of the specimen, together with representative ED patterns taken from different sample areas, are displayed in Fig. 5.
PV observations indicated that individual grains had a pseudo-hexagonal morphology (see Fig. 5a), with typical diameters of nearly 40 nm. The PV HR-TEM image in Fig. 5b unambiguously confirmed the grain hexagonal structure, with predominantly exposed (-1010), (1-100) and (0-110) facets. Furthermore, CS data (Fig. 5c) evidenced an epitaxial, columnar, c-oriented hematite growth, with the following relationship: [0001] α-Fe2O3//[0001] Al2O3 and (01-10) α-Fe2O3//(01-10) Al2O3.
The ED pattern in Fig. 5d left, imaged along the [2-1-10] zone axis orientation, is a superposition of the deposit and substrate structure, where the crystal phase of the former can be indexed using the space group and unit cell parameters of α-Fe2O3 [a = 0.503 nm, c = 1.374 nm, rhombohedral R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c(167)].2,35,43–45 Nevertheless, several ED images exhibited a more complex pattern, with various superstructure spots (compare Fig. 5d, right). A CS HR-TEM image taken from such a region is shown in Fig. 5e. The observed HR-TEM contrast and ED data can be likely traced back to the presence of regular twinnings within the α-Fe2O3 phase. A schematic view of the right-hand ED pattern, taking into account a twin structure over the (2-1-10) plane, is displayed in the central panel of Fig. 5d, where dots and squares correspond to two different α-Fe2O3 orientations sharing a common (0001) plane, whereas empty circles represent spots attributable to double diffraction phenomena.
c(167)].2,35,43–45 Nevertheless, several ED images exhibited a more complex pattern, with various superstructure spots (compare Fig. 5d, right). A CS HR-TEM image taken from such a region is shown in Fig. 5e. The observed HR-TEM contrast and ED data can be likely traced back to the presence of regular twinnings within the α-Fe2O3 phase. A schematic view of the right-hand ED pattern, taking into account a twin structure over the (2-1-10) plane, is displayed in the central panel of Fig. 5d, where dots and squares correspond to two different α-Fe2O3 orientations sharing a common (0001) plane, whereas empty circles represent spots attributable to double diffraction phenomena.
PV and CS TEM data suggest c-oriented, randomly distributed grains with two different orientation variants A and B, related to each other by a 60° rotation along the [0001] axis. A model for such a kind of growth is presented in Fig. 6. According to the proposed model, the difference between the two possibilities can be detected only in CS observations. Indeed, when the twin variants overlap, the structure observed in HR-TEM and ED patterns is expected to appear. Conversely, in the case of PV imaging, this rotation cannot be detected (see the top panel in Fig. 6).
 | ||
| Fig. 6 Atomic model of α-Fe2O3, grown epitaxially on Al2O3 (0001), presented in two orthogonal view directions corresponding to PV (top) and CS (bottom) TEM observations. | ||
In order to confirm the proposed twinned growth, HAADF-STEM imaging of the interface region was carried out. HAADF-STEM is mass-thickness sensitive, having image contrast that scales with the atomic number Z∼1.7. Since it is an incoherent imaging technique, diffraction contrast will not hinder the interpretation of the images, like in the case of HR-TEM. Fig. 7 shows representative high resolution HAADF-STEM images of a twinned area (Fig. 7a) and twin boundary (Fig. 7b). The structure models are overlaid, and match perfectly with the acquired images, confirming thus the validity of the proposed twinning model.
3.4 Optical properties
Special attention was finally devoted to investigating the combined influence of F-doping and morphology/phase composition on the system optical properties, in order to better evaluate the suitability of the above materials for eventual optoelectronic or photocatalytic applications. In particular, as also reported for other metal oxides, oxygen replacement by fluorine can shift the absorption edge, affect the recombination between photogenerated electrons and holes, passivate defect states, and impact the system resistivity modifying carrier concentration and mobility.9,11–16,19–21,38Fig. 8 displays the optical absorption spectra of iron oxide-based deposits grown at different temperatures. As can be observed, all specimens show a strong absorption for wavelengths lower than 600 nm, responsible for their red-to-yellow color.46–48 Under the assumption of a direct allowed transition, the following band-gap values were derived from Tauc plots in Fig. 8: 2.52 eV, 2.22 eV and 2.16 eV for specimens grown at 200, 300 and 400 °C, respectively. These values are significantly blue-shifted with respect to literature data for iron(III) oxides, that are typically close to 2.0 eV.5,46,49 A similar finding suggests that, beside phase composition, even other parameters appreciably affect the system optical properties. More specifically, the obtainment of band-gap values appreciably higher than literature ones can be traced back to a modified carrier concentration in Fe2O3 conduction/valence bands when oxygen vacancies are saturated by fluorine.9,19,20,50 This explanation also accounts for the progressive increase of band-gap values at the lowest deposition temperatures that, according to Fig. 1 and 2, result in a higher F-content in the obtained systems.
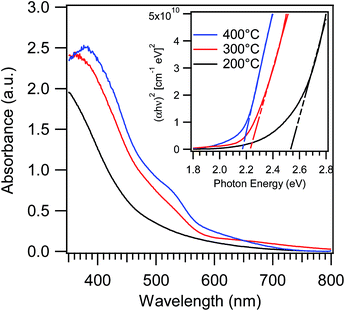 | ||
| Fig. 8 Optical absorption spectra of iron oxide samples grown at 200, 300 and 400 °C. The inset displays the corresponding Tauc plots. | ||
4. Conclusions
A PE-CVD approach to Fe2O3-based nanostructures on Al2O3(0001) single crystal substrates has been reported. The obtained iron oxide nanomaterials were in situ doped with fluorine thanks to the use of a fluorinated molecular compound, Fe(hfa)2TMEDA, acting as a single-source precursor for both Fe and F. Controlled variations of the deposition temperature directly impacted both the system chemical composition and crystalline phase. In particular, a γ-Fe2O3 to α-Fe2O3 phase transition was observed upon going from 200 to 300 °C, whereas highly oriented hematite nanocolumns were epitaxially grown at 400 °C. A detailed structural and morphological investigation enabled to obtain a deep insight on the interrelations between processing parameters and composition, structure/morphology and optical properties of the target systems. The proposed fabrication process paves the way to the development of iron oxide nanosystems for various applications, in particular in the fields of optoelectronics and photocatalysis, for which control of fluorine content, nano-organization, and optical properties is expected to result in a parallel tuning of functional performances. Further developments of the current research will concern the influence of F-doping on the magnetic properties of Fe2O3. Following our recent paper on the magnetic behavior of β- and ε-Fe2O3,51 future efforts will be specifically focused on the investigation of F-doped α- and γ-Fe2O3.Acknowledgements
The authors kindly acknowledge the financial support under the FP7 project “SOLAROGENIX” (NMP4-SL-2012-310333) as well as from Padova University ex-60% 2012-2013-2014, grant no. CPDR132937/13 (SOLLEONE), and Regione Lombardia-INSTM ATLANTE projects. Thanks are also due to Dr D. Bekermann (Padova University, Italy) for technical and synthetic assistance. S.T. gratefully acknowledges the FWO for a post-doctoral fellowship and for project number G004613N. This work was supported by funding from the European Research Council under the Seventh Framework Program (FP7), ERC grant no. 246791 – COUNTATOMS.Notes and references
- L. Machala, J. Tuček and R. Zbořil, Chem. Mater., 2011, 23, 3255 CrossRef CAS
.
- M. Lubbe, A. M. Gigler, R. W. Stark and W. Moritz, Surf. Sci., 2010, 604, 679 CrossRef PubMed
.
- D. Barreca, G. Carraro, A. Gasparotto, C. Maccato, F. Rossi, G. Salviati, M. Tallarida, C. Das, F. Fresno, D. Korte, U. Lavrenčič Štangar, M. Franko and D. Schmeisser, ACS Appl. Mater. Interfaces, 2013, 5, 7130 CAS
.
- G. Carraro, C. Maccato, A. Gasparotto, T. Montini, S. Turner, O. I. Lebedev, V. Gombac, G. Adami, G. Van Tendeloo, D. Barreca and P. Fornasiero, Adv. Funct. Mater., 2014, 24, 372 CrossRef CAS
.
- J. D. Desai, H. M. Pathan, S. K. Min, K. D. Jung and O. S. Joo, Appl. Surf. Sci., 2006, 252, 2251 CrossRef CAS PubMed
.
- P. Tartaj, M. P. Morales, T. Gonzalez-Carreño, S. Veintemillas-Verdaguer and C. J. Serna, Adv. Mater., 2011, 23, 5243 CrossRef CAS
.
- B. L. Lv, Z. Y. Liu, H. Tian, Y. Xu, D. Wu and Y. H. Sun, Adv. Funct. Mater., 2010, 20, 3987 CrossRef CAS
.
- K. Karthikeyan, S. Amaresh, S. N. Lee, V. Aravindan and Y. S. Lee, Chem.–Asian J., 2014, 9, 852 CrossRef CAS PubMed
.
- D. Barreca, G. Carraro, A. Gasparotto, C. Maccato, C. Sada, A. P. Singh, S. Mathur, A. Mettenbörger, E. Bontempi and L. E. Depero, Int. J. Hydrogen Energy, 2013, 38, 14189 CrossRef CAS PubMed
.
- G. Carraro, D. Barreca, D. Bekermann, T. Montini, A. Gasparotto, V. Gombac, C. Maccato and P. Fornasiero, J. Nanosci. Nanotechnol., 2013, 13, 4962 CrossRef CAS PubMed
.
- G. Carraro, D. Barreca, E. Comini, A. Gasparotto, C. Maccato, C. Sada and G. Sberveglieri, CrystEngComm, 2012, 14, 6469 RSC
.
- H. Seo, L. R. Baker, A. Hervier, J. Kim, J. L. Whitten and G. A. Somorjai, Nano Lett., 2011, 11, 751 CrossRef CAS PubMed
.
- A. Gasparotto, D. Barreca, D. Bekermann, A. Devi, R. A. Fischer, P. Fornasiero, V. Gombac, O. I. Lebedev, C. Maccato, T. Montini, G. Van Tendeloo and E. Tondello, J. Am. Chem. Soc., 2011, 133, 19362 CrossRef CAS PubMed
.
- C. O'Keeffe, P. Gannon, P. Gilson, A. Kafizas, I. P. Parkin and R. Binions, Thin Solid Films, 2013, 537, 131 CrossRef PubMed
.
- Y.-J. Choi and H.-H. Park, J. Mater. Chem. C, 2014, 2, 98 RSC
.
- A. Kafizas, N. Noor, P. Carmichael, D. O. Scanlon, C. J. Carmalt and I. P. Parkin, Adv. Funct. Mater., 2014, 24, 1758 CrossRef CAS
.
- H. F. Liang and R. G. Gordon, J. Mater. Sci., 2007, 42, 6388 CrossRef CAS PubMed
.
- I. Akyuz, S. Kose, E. Ketenci, V. Bilgin and F. Atay, J. Alloys Compd., 2011, 509, 1947 CrossRef CAS PubMed
.
- J. Santos-Cruz, G. Torres-Delgado, R. Castanedo-Perez, C. I. Zuniga-Romero and O. Zelaya-Angel, Thin Solid Films, 2007, 515, 5381 CrossRef CAS PubMed
.
- R. Gonzalez-Hernandez, A. I. Martinez, C. Falcony, A. A. Lopez, M. I. Pech-Canul and H. M. Hdz-Garcia, Mater. Lett., 2010, 64, 1493 CrossRef CAS PubMed
.
- C.-l. Song, J. Wang, M.-l. Zeng, J.-q. Zhu, Y. Liu, G. Xu and G.-r. Han, J. Sol-Gel Sci. Technol., 2013, 68, 121 CrossRef CAS
.
- X. Noirfalise, T. Godfroid, G. Guisbiers and R. Snyders, Acta Mater., 2011, 59, 7521 CrossRef CAS PubMed
.
- N. S. Chaudhari, S. S. Warule, S. Muduli, B. B. Kale, S. Jouen, B. Lefez, B. Hannoyer and S. B. Ogale, Dalton Trans., 2011, 40, 8003 RSC
.
- M. Kul, A. S. Aybek, E. Turan, M. Zor and S. Irmak, Sol. Energy Mater. Sol. Cells, 2007, 91, 1927 CrossRef CAS PubMed
.
- F. Bertram, C. Deiter, K. Pflaum, M. Suendorf, C. Otte and J. Wollschlager, J. Appl. Phys., 2011, 110, 102208 CrossRef PubMed
.
- T. Fujii, F. M. F. de Groot, G. A. Sawatzky, F. C. Voogt, T. Hibma and K. Okada, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 3195 CrossRef CAS
.
- S. Maheswaran, S. Thevuthasan, F. Gao, V. Shutthanandan, C. M. Wang and R. J. Smith, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 7 CrossRef
.
- D. Barreca, G. Carraro, A. Devi, E. Fois, A. Gasparotto, R. Seraglia, C. Maccato, C. Sada, G. Tabacchi, E. Tondello, A. Venzo and M. Winter, Dalton Trans., 2012, 41, 149 RSC
.
- D. Barreca, A. Gasparotto, E. Tondello, C. Sada, S. Polizzi and A. Benedetti, Chem. Vap. Deposition, 2003, 9, 199 CrossRef CAS
.
- D. Briggs and M. P. Seah, Practical Surface Analysis, Wiley, New York, 1990 Search PubMed
.
- N. A. M. Barakat, J. Mater. Sci., 2012, 47, 6237 CrossRef CAS
.
- G. Carraro, A. Gasparotto, C. Maccato and D. Barreca, Surf. Sci. Spectra, 2013, 20, 9 CrossRef
.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, Perkin Elmer Corporation, Eden Prairie, MN, USA, 1992 Search PubMed
.
- P. Li, E. Y. Jiang and H. L. Bai, J. Phys. D: Appl. Phys., 2011, 44, 075003 CrossRef
.
- S. I. Yi, Y. Liang, S. Thevuthasan and S. A. Chambers, Surf. Sci., 1999, 443, 212 CrossRef CAS
.
- Y. Gao, Y. J. Kim, S. A. Chambers and G. Bai, J. Vac. Sci. Technol., A, 1997, 15, 332 CAS
.
- P. F. Fulvio, S. S. Brown, J. Adcock, R. T. Mayes, B. K. Guo, X. G. Sun, S. M. Mahurin, G. M. Veith and S. Dai, Chem. Mater., 2011, 23, 4420 CrossRef CAS
.
- S. Sumitsawan, J. Cho, M. L. Sattler and R. B. Timmons, Environ. Sci. Technol., 2011, 45, 6970 CrossRef CAS PubMed
.
- D. Barreca, D. Bekermann, E. Comini, A. Devi, R. A. Fischer, A. Gasparotto, M. Gavagnin, C. Maccato, C. Sada, G. Sberveglieri and E. Tondello, Sens. Actuators, B, 2011, 160, 79 CrossRef CAS PubMed
.
- Pattern no. 39-1346, JCPDS, 2000.
- Pattern no. 33-0664, JCPDS, 2000.
- D. Barreca, A. Devi, R. A. Fischer, D. Bekermann, A. Gasparotto, M. Gavagnin, C. Maccato, E. Tondello, E. Bontempi, L. E. Depero and C. Sada, CrystEngComm, 2011, 13, 3670 RSC
.
- A. Barbier, O. Bezencenet, C. Mocuta, J. B. Moussy, H. Magnan, N. Jedrecy, M. J. Guittet and M. Gautier-Soyer, Mater. Sci. Eng., B, 2007, 144, 19 CrossRef CAS PubMed
.
- C. M. Wang, S. Thevuthasan, F. Gao, D. E. McCready and S. A. Chambers, Thin Solid Films, 2002, 414, 31 CrossRef CAS
.
- I. J. Lee, J. Y. Kim, C. Yu, C. H. Chang, M. K. Joo, Y. P. Lee, T. B. Hur and H. K. Kim, J. Vac. Sci. Technol., A, 2005, 23, 1450 CAS
.
- Y. P. He, Y. M. Miao, C. R. Li, S. Q. Wang, L. Cao, S. S. Xie, G. Z. Yang, B. S. Zou and C. Burda, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 125411 CrossRef
.
- D. M. Sherman and T. D. Waite, Am. Mineral., 1985, 70, 1262 CAS
.
- D. A. Wheeler, G. M. Wang, Y. C. Ling, Y. Li and J. Z. Zhang, Energy Environ. Sci., 2012, 5, 6682 CAS
.
- B. Gilbert, J. E. Katz, J. D. Denlinger, Y. D. Yin, R. Falcone and G. A. Waychunas, J. Phys. Chem. C, 2010, 114, 21994 CAS
.
- E. Burstein, Phys. Rev., 1954, 93, 632 CrossRef CAS
.
- G. Carraro, D. Barreca, C. Maccato, E. Bontempi, L. E. Depero, C. de Julián Fernández and A. Caneschi, CrystEngComm, 2013, 15, 1039 RSC
.
| This journal is © The Royal Society of Chemistry 2014 |