Magnetic nanocatalyst for the synthesis of maleimide and phthalimide derivatives†
Abstract
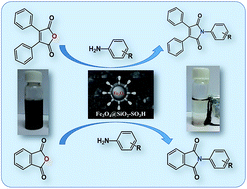
An efficient and green protocol for the synthesis of N-aryl maleimide and phthalimide derivatives has been developed. The high efficiency of the catalyst was observed due to the homogeneous distribution of the nanoparticles. The catalyst was fully characterised by physicochemical methods such as IR spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction, dynamic light scattering (DLS), energy dispersive X-ray spectrum and zeta potential measurement techniques. The ease of separation of the catalyst from the reaction mixture and its high activity are eco-friendly attributes of this system.

 Please wait while we load your content...
Please wait while we load your content...