Synthesis of β-ketoesters from renewable resources and Meldrum's acid†
Rafael C. Brinkerhoffa,
Hernan F. Tarazonaa,
Patrick M. de Oliveiraa,
Darlene C. Floresa,
Caroline Da R. Montes D'Ocab,
Dennis Russowskyb and
Marcelo G. Montes D'Oca*a
aUniversidade Federal do Rio Grande, Laboratório Kolbe de Síntese Orgânica, Rio Grande-RS, Brazil. E-mail: dqmdoca@furg.br; Tel: +55 5332336964
bUniversidade Federal do Rio Grande do Sul-UFRGS, Laboratório de Síntese Orgânica, Porto Alegre-RS, Brazil
First published on 23rd September 2014
Abstract
β-Ketoesters are valuable building blocks for the synthesis of compounds with different biological activities. In this study, a series of fatty β-ketoesters were obtained from fatty acids and Meldrum's acid using N,N-dicyclohexylcarbodiimide and dimethylaminopyridine. In addition, we demonstrate for the first time the synthesis of new fatty β-ketoesters from oleic (cis-C18:1), elaidic (trans-C18:1), ricinoleic (cis-C18:1, 12-OH), linoleic (cis,cis-C18:2), and linolenic (cis,cis,cis-C18:3) acids in good yields.
β-Ketoesters are extremely important and versatile organic compounds with a wide range of applications, including alpha-halogenation and alpha-azidation,1 synthesis of thiazoles and thiophenes,2 and use in multicomponent reactions.1–6 Using β-ketoesters as the building blocks in multicomponent reactions yields several different structures based on the dihydropyridinone, tetrahydropyridine, or dihydropyridine skeleton, creating an extensive library of compounds with various biological activities.4–7
According to the literature, the transesterification process for the synthesis of β-ketoesters in solvent-free conditions without the use of catalysis results in high yields when using excess alcohol at a high temperature.3 Primary, secondary, and tertiary alcohols have been tested in the presence of molecular sieves, resulting in good β-ketoester yields.8 The catalysts in the transesterification reaction vary and include new silica-based hybrid materials,9 triethylamine,10 boric acid,11 and triphenylphosphine.12
Claisen condensation is a classic method of β-ketoester synthesis. The condensation of the acid chlorides and esters catalysed by titanium tetrachloride and N-methylimidazole produces various β-ketoesters in good yields.13 Crossed Claisen condensation reactions of ketene silyl acetals and methyl esters catalysed by sodium hydroxide have also resulted in good yields of various β-ketoesters.14,15
An important route for the synthesis of β-ketoesters is acylation of Meldrum's acid followed by a reaction with an alcohol. Oikawa et al.16 showed that the acylation of various acyl chlorides with Meldrum's acid yielded the corresponding acyl Meldrum's acids, which readily underwent alcoholysis with methanol, ethanol, tert-butyl alcohol, benzyl alcohol, and trichloroethanol to produce various β-ketoesters.
C-acylation of Meldrum's acid by N-protected amino acids, using isopropenyl chloroformate (IPCF) or dicyclohexylcarbodiimide (DCC) and dimethylaminopyridine (DMAP) as the condensing agent, has been performed, and δ-amino-β-ketoesters have been obtained in high yields.17
In a continuation of our studies developing new fatty acid compounds,18–20 we investigated the synthesis of β-ketoesters through acylation reactions of Meldrum's acid with different families of saturated and unsaturated carboxylic acids. In addition, we report for the first time the synthesis of new fatty β-ketoesters from oleic (cis-C18:1), elaidic (trans-C18:1), ricinoleic (cis-C18:1, 12-OH), linoleic (cis,cis-C18:2), and linolenic (cis,cis,cis-C18:3) acids.
Initially, experiments using palmitic acid (1e) as a model with thionyl chloride and pyridine in the presence of Meldrum's acid (2) were undertaken according to the literature (Scheme 1).16 However, after a long reaction time, the fatty β-ketoester 4e was obtained in a poor yield (43% from 1e).
Therefore, afterward, we examined the use of DMAP and DCC as a coupling agent to synthesise the fatty β-ketoesters from Meldrum's acid and the fatty acids.
Experiments were carried out using palmitic acid (1e) as the model, along with Meldrum's acid (2), DCC, DMAP, and pyridine at room temperature under a nitrogen atmosphere. The enol derivative 3e was isolated and used without previous purification. Then, β-ketoester 4e was obtained from a reaction of the respective enol 3e and methanol in an acid catalyst at reflux for 12 h. The purified product 4e was obtained in a 65% yield from 1e (Scheme 2).
 | ||
| Scheme 2 Synthesis of fatty β-ketoester 4e from Meldrum's acid (2) and fatty acid 1e using DMAP and DCC as the coupling agents (Method A). | ||
During the purification of β-ketoester 4e by chromatography, a significant amount of methyl palmitate was obtained from what could only have been an acid-catalysed esterification reaction of unreacted palmitic acid (1e) from the acylation step with methanol.
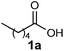
To investigate the acylation reaction with various fatty acids (C6:0, C8:0, C10:0, C12:0, C18:0, cis-C18:1, trans-C18:1, C18:2, C18:3), we used the same protocol to obtain a wide range of fatty β-ketoesters, namely, 4a–d and 4f–j. However, the protocol resulted in the β-ketoester derivatives 4a–j with saturated and unsaturated alkyl chains in moderate yields (Method A, Table 1).
Next we examined a modified methodology that includes a two-step process. First, the effect of adding palmitic acid (1e) over DCC in the absence of Meldrum's acid (2) was studied. It has been proposed that the condensation reaction between DCC and carboxylic acids initially forms the O-acylisourea intermediate.21 Indeed, in our experiments, after adding fatty acid over DCC, fatty O-acylisourea promptly formed. Next, adding 2.0 equiv. of Meldrum's acid (2) in dichloromethane and pyridine under the same experimental conditions resulted in the formation of the enol derivative 3e (Scheme 3). Subsequently, β-ketoester 4e was obtained from the reaction of enol 3e and methanol in an acid catalyst at reflux. Therefore, the modified protocol resulted in an increased yield (74–84%) of the β-ketoester derivatives 4a–j (Method B,22 Table 1).
 | ||
| Scheme 3 Synthesis of fatty β-ketoesters 4a–j from Meldrum's acid (2) and the fatty acids 1a–j (Method B). | ||
In this context, the moderated yield of fatty β-ketoesters 4a–j obtained using the one-pot method (Method A) must be attributed to the consumption of DCC (the coupling agent) from the nucleophilic attack of Meldrum's acid (2).
In Method B, the addition of the fatty acid over DCC in the absence of Meldrum's acid, leading to the consumption of the complete fatty acid and the nucleophilic attack of Meldrum's acid (2) on the preformed fatty O-acylisourea, appears to have been crucial for the increased yield (Scheme 3).
Finally, the β-ketoester 4k derivative from ricinoleic acid was synthesised (Scheme 4). Ricinoleic acid or 12-hydroxy-9-cis-octadecenoic acid is the major constituent (80–90%) of castor oil (Ricinus communis)23 and is an uncommon fatty acid that contains a double bond and a hydroxyl group in the chain. Compound 4k was synthesised by Method B at room temperature under a nitrogen atmosphere using Meldrum's acid (2) and ricinoleic acid (cis-C18:1, 12-OH, 1k), which was obtained from the castor oil or via castor oil biodiesel hydrolysis. The purified product 4k (ref. 28) was obtained in a 75% yield from 1k.
 | ||
| Scheme 4 Synthesis of fatty β-ketoester 4k from Meldrum's acid (2) and ricinoleic acid (1k, Method B). | ||
Conclusions
In conclusion, we used a simple method to develop fatty β-ketoesters using Meldrum's acid and a wide range of fatty chains of saturated, unsaturated, and hydroxylated acids. We are currently in the process of synthesizing a series of new fatty acid dihydropyrimidinones with different structural arrangements using Biginelli's multicomponent protocol29 in the presence of the new fatty β-ketoesters from renewable resources.Acknowledgements
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Apoio à Pesquisa do Estado do Rio Grande do Sul (FAPERGS/PRONEM) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support.Notes and references
- M. J. Galligan, R. Akula and H. Ibrahim, Org. Lett., 2014, 16, 600 CrossRef CAS PubMed.
- L. C. Luo, L. L. Meng, Q. Sun, Z. M. Ge and R. T. Li, Tetrahedron Lett., 2014, 55, 259 CrossRef CAS PubMed.
- G. B. D. Rao, B. N. Acharya and M. P. Kaushik, Tetrahedron Lett., 2013, 54, 6644 CrossRef PubMed.
- L. Liu, R. Sarkisian, Y. M. Deng and H. Wang, J. Org. Chem., 2013, 78, 5751 CrossRef CAS PubMed.
- D. Russowsky, R. F. S. Canto, S. A. A. Sanches, M. G. M. D'Oca, A. Fátima, R. A. Pilli, L. K. Konhn, M. A. Antônio and J. E. Carvalho, Bioorg. Chem., 2006, 34, 173 CrossRef CAS PubMed.
- A. Crespo, A. El Maatougui, P. Biagini, J. Azuaje, A. Coelho, J. Brea, M. I. Loza, M. I. Cadavid, X. Garcia-Mera, H. Gutierrez-de-Teran and E. Sotelo, ACS Med. Chem. Lett., 2013, 4, 1031 CrossRef CAS PubMed.
- D. Bonne, Y. Coquerel, T. Constantieux and J. Rodriguez, Tetrahedron: Asymmetry, 2010, 21, 1085 CrossRef CAS PubMed.
- L. I. Koval, V. I. Dzyuba, O. L. Ilnitska and V. I. Pekhnyo, Tetrahedron Lett., 2008, 49, 1645 CrossRef CAS PubMed.
- G. Sathicq, L. Musante, G. Romanelli, G. Pasquale, J. Autino, H. Thomas and P. Vazquez, Catal. Today, 2008, 133, 455 CrossRef PubMed.
- O. Mhasni and F. Rezgui, Tetrahedron, 2011, 67, 6322 CrossRef CAS PubMed.
- G. C. M. Kondaiah, L. A. Reddy, K. S. Babu, V. M. Gurav, K. G. Huge, R. Bandichhor, P. P. Reddy, A. Bhattacharya and R. V. Anand, Tetrahedron Lett., 2008, 49, 106 CrossRef CAS PubMed.
- J. S. Yadav, B. V. S. Reddy, A. D. Krishna, C. S. Reddy and A. V. Narsaiah, J. Mol. Catal. A: Chem., 2007, 261, 93 CrossRef CAS PubMed.
- T. Misaki, R. Nagase, K. Matsumoto and Y. Tanabe, J. Am. Chem. Soc., 2005, 127, 2854 CrossRef CAS PubMed.
- A. Iida, K. Takai, T. Okabayashi, T. Misaki and Y. Tanabe, Chem. Commun., 2005, 3171 RSC.
- K. Takai, Y. Nawate, T. Okabayashi, H. Nakatsuji, A. Iida and Y. Tanabe, Tetrahedron, 2009, 65, 5596 CrossRef CAS PubMed.
- Y. Oikawa, K. Sugano and O. Yonemitsu, J. Org. Chem., 1978, 43, 2087 CrossRef CAS.
- B. Li and R. W. Franck, Bioorg. Med. Chem. Lett., 1999, 9, 2629 CrossRef CAS.
- C. R. M. D'Oca, T. Coelho, T. G. Marinho, C. R. L. Hack, R. C. Duarte, P. A. da Silva and M. G. M. D'Oca, Bioorg. Med. Chem. Lett., 2010, 20, 5255 CrossRef PubMed.
- R. C. Duarte, R. Ongaratto, L. A. Piovesan, V. R. de Lima, V. Soldi, A. A. Merlo and M. G. M. D'Oca, Tetrahedron Lett., 2012, 53, 2454 CrossRef CAS PubMed.
- M. O. Rodrigues, J. B. Cantos, C. R. M. D'Oca, K. L. Soares, T. S. Coelho, L. A. Piovesan, D. Russowsky, P. A. Silva and M. G. M. D'Oca, Bioorg. Med. Chem., 2013, 21, 6910 CrossRef CAS PubMed.
- H. Wiener and C. Gilon, J. Mol. Catal., 1986, 37, 45 CrossRef CAS.
- General procedure for synthesis of β-ketoesters 4a–k (Method B): fatty acid 1a–k (1 mmol), DCC (1.1 mmol), and DMAP (0.3 mmol) were dissolved in dichloromethane (15 mL) and stirred for 30 min. Then, Meldrum's acid (2 mmol) and pyridine (3.6 mmol) were dissolved in dichloromethane (10 mL) and added dropwise. The mixture was stirred for 24 h at room temperature. The solid dicyclohexylurea formed was removed by filtration, and the filtrated organic layer was washed with an acid solution (10% HCl, 3 × 25 mL) and dried over Mg2SO4. The solvent was removed by reduced pressure and the respective enol 3a–k was obtained. The crude product was dissolved in methanol (25 mL), and 5 drops H2SO4 were added to the mixture, which was refluxed for 12 h. The solvent was removed by reduced pressure, the residue was dissolved in dichloromethane (25 mL), and the organic layer was washed with distilled H2O (3 × 25 mL) and dried over Mg2SO4. The solvent was removed by reduced pressure and the residue obtained was purified by flash column chromatography on silica gel, eluent, and either hexane/diethyl ether (97
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford β-ketoesters 4a–j or hexane/ethyl acetate (8
3) to afford β-ketoesters 4a–j or hexane/ethyl acetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to obtain β-ketoester 4k.
2) to obtain β-ketoester 4k. - G. Lakshminarayana, M. M. Paulose and N. B. Kumari, J. Am. Oil Chem. Soc., 1984, 61, 1871 CrossRef CAS.
- For 4g: 81%, colorless oil. FT-IR (NaCl, film, ν = cm−1) 3008.9, 2926.0, 2854.6, 1747.5, 1716.6, 1460.1, 1217.1, 758.1. 1H NMR (CDCl3): δ (ppm) 0.86 (t, 3H, J = 6.0 Hz, CH3), 1.26 (m, 20H, CH2), 1.57 (t, 2H, J = 6.0 Hz, CH2 β-carbonyl), 1.99 (m, 4H, CH2 allylic), 2.51 (t, 2H, J = 6.0 Hz, CH2 α carbonyl), 3.43 (s, 2H, CH2 bis-α carbonyl), 3.71 (s, 3H, OCH3), 5.32 (m, 2H, 2CH vinylic). 13C NMR (CDCl3) δ (ppm) 14.0, 22.6, 23.4, 24.2, 27.1, 27.2, 28.9, 29.0, 29.2, 29.3, 29.5, 29.6, 29.7, 31.8, 43.0, 48.9, 52.3, 129.6, 129.9, 167.6, 202.8.
- For 4h: 78%, colorless oil. FT-IR (NaCl, film, ν = cm−1) 3020.5, 2927.9, 2854.6, 11745.5, 1716.6, 1460.1, 1215.1, 758.0. 1H NMR (CDCl3): δ (ppm) 0.88 (t, 3H, J = 6.0 Hz, CH3), 1.26 (m, 20H, CH2), 1.59 (m, 2H, CH2 β carbonyl), 1.96 (m, 4H, CH2 allylic), 2.53 (t, 2H, J = 6.0 Hz, CH2 α carbonyl), 3.45 (s, 2H, CH2 bis-α carbonyl), 3.74 (s, 3H, OCH3), 5.38 (m, 2H, CH vinylic). 13C NMR (CDCl3) δ (ppm) 14.1, 22.6, 23.4, 28.9, 29.0, 29.2 (2C), 29.3, 29.5, 29.6, 29.7, 31.9, 32.5, 32.6, 43.1, 49.0, 52.3, 130.2, 130.5, 167.7, 202.9.
- For 4i: 80%, colorless oil. FT-IR (NaCl, film, ν = cm−1), 3014.0, 2927.1, 2856.3, 1747.2, 1716.1, 1462.7, 1438.1, 1215.0 1H NMR (CDCl3): δ (ppm) 0.89 (t, 3H, J = 6.0 Hz, CH3), 1.30 (m, 14H, CH2), 1.59 (m, 2H, CH2 β-carbonyl), 2.05 (q, J = 6.0 Hz, 4H, CH2 allylic), 2.53 (t, 2H, J = 6.0 Hz, CH2 α-carbonyl), 2.77 (t, 2H, J = 6.0 Hz, CH2 bis-allylic), 3.45 (s, 2H, CH2 bis -α carbonyl), 3.74 (s, 3H, OCH3), 5.34 (m, 4H, CH vinylic). 13C NMR (CDCl3) δ (ppm) 14.0, 22.5, 23.3, 25.5, 27.1 (2C), 28.9, 29.0, 29.2, 29.3, 29.5, 31.4, 43.0, 48.9, 52.3, 127.8, 128.0, 129.9, 130.1, 167.6, 202.8.
- For 4j: 80%, colorless oil. FT-IR (NaCl, film, ν = cm−1), 3455.0, 2931.0, 2857.0, 1746.0, 1709.0, 1435.0, 1312.0, 1258.0, 1161.0, 1075.0, 971.0. 1H NMR (CDCl3): δ (ppm) 0.90 (t, 3H, J = 7.5 Hz, CH3), 1.22 (m, 8H, CH2), 1.51 (m, 2H, CH2 β-carbonyl), 1.98 (m, 4H, CH2 allylic), 2.46 (t, 2H, J = 7.5 Hz, CH2 α-carbonyl), 2.73 (t, 4H, J = 6.0 Hz, CH2 bis-allylic), 3.38 (s, 2H, CH2 bis-α carbonyl), 3.66 (s, 3H, OCH3), 5.28 (m, 6H, CH vinylic). 13C NMR (CDCl3) δ (ppm) 14.2, 20.4, 23.3, 25.4, 25.5, 27.1, 28.9, 29.0, 29.2, 29.5, 42.9, 48.9, 52.2, 127.0, 127.6, 128.1, 128.2, 130.1, 131.8, 167.7, 202.8.
- For 4k: 75%, colorless oil. FT-IR (NaCl, film, ν = cm−1), 3489.2, 3018.6, 2929.8, 2856.5, 1737.8, 1714.7, 1438.9, 1215.1. 1H NMR (CDCl3): δ (ppm) 0.81 (t, 3H, J = 6.0 Hz, CH3), 1.22 (m, 16H, CH2), 1.40 (m, 2H, CH2-α-OH), 1.52 (m, 2H, CH2 β-carbonyl), 1.97 (q, 2H, J = 6.0 Hz, CH2 allylic), 2.14 (t, 2H, J = 6.0 Hz, CH2 allylic-α-OH), 2.46 (t, 2H, J = 6.0 Hz, CH2 α-carbonyl), 3.38 (s, 2H, CH2 bis-α-carbonyl), 3.54 (q, 1H, J = 6.0 Hz, CH carbinolic), 3.67 (s, 3H, OCH3), 5.33 (m, 1H, CH vinylic), 5.48 (m, 1H, CH vinylic). 13C NMR (CDCl3) δ (ppm) 13.0, 21.6, 22.3, 24.7, 26.3, 27.9, 28.0, 28.1, 28.3, 28.5, 30.8, 34.3, 35.8, 42.0, 48.0, 51.3, 70.4, 124.2, 132.3, 166.7, 201.8.
- D. Russowsky, F. A. Lopes, V. S. S. da Silva, K. F. S. Canto, M. G. M. D'Oca and M. N. Godoi, J. Braz. Chem. Soc., 2004, 15, 165 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08986c |
| This journal is © The Royal Society of Chemistry 2014 |