Trimethylethoxysilane-modified super heat-resistant alumina aerogels for high-temperature thermal insulation and adsorption applications
Wenqin Wanga,
Zhihua Zhang*a,
Guoqing Zua,
Jun Shena,
Liping Zoua,
Ya Liana,
Bin Liub and
Fan Zhangb
aShanghai Key Laboratory of Special Artificial Microstructure Materials and Technology, Pohl Institute of Solid State Physics, Tongji University, Shanghai 200092, P.R. China. E-mail: wwqtj2014@163.com; zzhtj@tongji.edu.cn; Fax: +86 21 65986071; Tel: +86 21 65986071
bAerospace Research Institute of Special Material and Processing Technology, Beijing 100074, P.R. China
First published on 6th October 2014
Abstract
The study of heat resistance of alumina aerogels has drawn great attention because of their high-temperature thermal insulation and catalyst applications. However, the main problem for the synthesis of heat-resistant alumina aerogels is their limited heat resistance and the drastic decrease of specific surface area upon heat treatment. Herein, trimethylethoxysilane (TMEO)-modified alumina aerogels are prepared by introduction of TMEO during sol–gel and supercritical fluid drying (SCFD) process. The introduced TMEO not only restricts the condensation of surface hydroxyl groups during aging and drying but also produces small silica particles on the alumina surface at high temperatures which inhibits the crystal growth upon heat treatment. Hence, the heat resistance of alumina aerogels is significantly enhanced after TMEO modification. The optimized TMEO-modified alumina aerogel has no cracks and little shrinkage during high-temperature SCFD, and shows no shrinkage and a high specific surface area of 147 m2 g−1 after heat treatment at 1200 °C. The TMEO-modified alumina aerogel possesses enhanced adsorption performance for gentian violet after firing at 1200 °C and shows low thermal conductivities of 0.13 and 0.18 W m−1 K−1 at 800 and 1000 °C, respectively. This may significantly contribute to their high-temperature applications such as thermal insulation, adsorption, catalysts, catalyst supports, etc.
1. Introduction
Owing to their high porosity, high specific surface area, extremely low density and better heat resistance than silica aerogels, alumina aerogels have attracted considerable academic and industrial attention in the applications of high-temperature thermal insulation and catalysts.1–3 An attractive method to prepare alumina aerogel is the sol–gel route from hydrated alumina salts or aluminum alkoxides.4–18 The alumina aerogels derived from aluminum alkoxides show better thermal stability than those derived from hydrated alumina salts. However, the alumina aerogels are susceptible to cracking and shrinking during aging and drying and their heat resistance still could not meet the needs, which badly limits their high-temperature applications.4 Many research works have been done in order to enhance the heat resistance of alumina aerogels, of which most attentions have been paid to alumina–silica composites.19–26 However, up to now, there are only few reports describing alumina aerogels with heat resistance above 1000 °C.4,26In this paper, we have developed a novel method to synthesis trimethylethoxysilane (TMEO)-modified alumina aerogels. After TMEO modification, the cracking and shrinking of alumina aerogels during drying and heat treatment are effectively inhibited and the specific surface areas at high temperatures are significantly enhanced. In this method, silica is introduced through TMEO modification during sol–gel and supercritical fluid drying (SCFD) process. During sol–gel process, TMEO would react with the hydroxyl groups on the alumina surface to form methyl siloxyl groups on the alumina surface, which reduces the amount of Al–OH and produces –Si–(CH3)3 on the alumina surface. Therefore, the condensation of the neighbouring –OH on the alumina surface during aging and drying is effectively restricted. The cracking and shrinking of alumina wet gels during aging and drying are effectively inhibited accordingly. During SCFD process, the alumina aerogels are further modified by partially hydrolyzed aluminum-tri-sec-butoxide (ASB) and TMEO. The obtained TMEO-modified alumina aerogels show super heat resistance (up to 1200 °C) and high specific surface areas at high temperatures, which may significantly contribute to their practical high-temperature applications such as thermal insulation, catalysts, catalyst supports. As a demonstration for functionality, we show that the obtained TMEO-modified alumina aerogel possesses enhanced adsorption performance for gentian violet after treatment as high as 1200 °C and owns low thermal conductivities of 0.13 and 0.18 W m−1 K−1 at 800 and 1000 °C, respectively.
2. Experimental section
2.1. Materials
Ethanol, distilled water, TMEO, acetone, aniline and HNO3 (68%) were purchased from sinopharm Chemical Reagent Corporation (China). ASB was obtained from LianYungang Lianlian Chemicals Corporation (China). All of the chemical reagents were used as received.2.2. Gel preparation with TMEO modification
The alumina wet gels were prepared according to an acetone–aniline in situ water formation (ISWF) method.26 This technique can realize slow in situ release of water through dehydration reaction of acetone and aniline, which leads to lower the hydrolysis and condensation of highly reactive metal alkoxide and thus transparent metal oxide gels with uniform microstructure could be obtained without using any chelating agents. It involves the following three steps. First, ASB was dissolved in ethanol and distilled water at 60 °C with stirring. After turning clear, the mixture was cooled to room temperature. Second, ethanol–diluted nitric acid was added into the mixture with stirring. Third, an appropriate amount of solution of distilled water, acetone, aniline and TMEO with a certain molar ratio was added into above mixture. The mixture was stirred for 10 min and then poured into a glass beaker where the gel would be formed. Table 1 details the sol–gel parameters for the synthesis of TMEO-modified alumina aerogels. The molar ratio of TMEO to alumina of the samples A-T0.45, A-T0.65, A-T0.8 and A-T1.1 are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.8, 1
6.8, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7, 1
4.7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8 and 1
3.8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 respectively.
2.8 respectively.
| Sample | Step one | Step two | Step three | |||||
|---|---|---|---|---|---|---|---|---|
| ASB (g) | EtOH (mL) | H2O (mL) | EtOH (mL) | HNO3 (mL) | Acetone (mL) | Aniline (mL) | TMEO (mL) | |
| A | 4.64 | 3.5 | 0.1 | 0.3 | 0.03 | 2.0 | 2.0 | 0 |
| A-T0.45 | 4.64 | 3.5 | 0.1 | 0.3 | 0.03 | 2.0 | 2.0 | 0.45 |
| A-T0.65 | 4.64 | 3.5 | 0.1 | 0.3 | 0.03 | 2.0 | 2.0 | 0.65 |
| A-T0.8 | 4.64 | 3.5 | 0.1 | 0.3 | 0.03 | 2.0 | 2.0 | 0.8 |
| A-T1.1 | 4.64 | 3.5 | 0.1 | 0.3 | 0.03 | 2.0 | 2.0 | 1.1 |
2.3. SCFD with ASB/TMEO modification
After aging and solvent exchange with ethanol for a few days, the wet gel was supercritically dried with ASB/TMEO modification. The wet gel was put into an autoclave containing certain amount of ethanol with partially hydrolyzed ASB and TMEO. The partially hydrolyzed ASB was prepared by dissolving ASB in the mixture of ethanol and distilled water at 60 °C with stirring for 5 minutes. The molar ratio of ASB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH
EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O was kept at 1
H2O was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.5
14.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6. The molar ratio of the added ASB to Al3+ in alumina aerogel was kept at 0.7. The molar ratio of the added modifier ASB to TMEO was 3.8
0.6. The molar ratio of the added ASB to Al3+ in alumina aerogel was kept at 0.7. The molar ratio of the added modifier ASB to TMEO was 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After it was sealed, ultra pure, dry nitrogen gas was flushed in the autoclave. The autoclave temperature was raised to 300 °C at a rate of 1 °C min−1 while the pressure rose and was controlled at ∼15 MPa. It was maintained at 300 °C and 15 MPa for 2 h, and the autoclave was then decompressed slowly at a rate of 30 kPa min−1. Finally, the system was cooled down to room temperature naturally and the aerogel was removed. The samples A and A-T0.8 after ASB/TMEO modification are denoted as A–A/T and A-T0.8–A/T respectively.
1. After it was sealed, ultra pure, dry nitrogen gas was flushed in the autoclave. The autoclave temperature was raised to 300 °C at a rate of 1 °C min−1 while the pressure rose and was controlled at ∼15 MPa. It was maintained at 300 °C and 15 MPa for 2 h, and the autoclave was then decompressed slowly at a rate of 30 kPa min−1. Finally, the system was cooled down to room temperature naturally and the aerogel was removed. The samples A and A-T0.8 after ASB/TMEO modification are denoted as A–A/T and A-T0.8–A/T respectively.
2.4. Characterization
The bulk density of alumina aerogels was determined by ρ = M/V where ρ, M and V are bulk density, mass and volume (obtained by V = πD2h/4 where D and h are diameter and height of the aerogel disk) of the aerogels respectively. The morphology of the sample was characterized by a transmission electron microscope (TEM, JEOL-1230, Japan). The nature of phases in the aerogels was analyzed by powder X-ray diffraction (XRD) on a Rigata/max-C diffractometer using the Cu-Kα radiation (DX-2700, Hao Yuan Instrument, China). Organic groups were investigated by a Fourier transform infrared spectroscope (FTIR, TENSOR27, Bruker, Germany). The pore size distribution and specific surface area were measured by a N2 adsorption analyzer (TriStar 3000, Quantachrome Instruments, USA) using the BET nitrogen adsorption/desorption technique. The absorption spectra of gentian violet solution in the presence of alumina aerogel was measured by a UV/VIS/NIR Spectrophotometer (V-570, JASCO, Japan). The high-temperature thermal conductivities are measured by a hot-wire thermal conductivity instrument (QTM-500, KEM, Japan).3. Results and discussion
3.1. The effect of TMEO modification on appearance during aging and drying
Unlike silica aerogels that shrink and crack little, alumina aerogels usually suffer much larger shrinking and cracking during aging and high-temperature SCFD.5,7 We found that the problem could be effectively resolved via TMEO modification. The morphologies of alumina wet gels and aerogels could be effectively influenced by the amount of the added TMEO. The appearances of the unmodified and TMEO-modified alumina wet gels are shown in Fig. 1. It is clearly seen that the unmodified alumina wet gel shows large shrinkage (∼15% in linear) and several cracks after aging for 2 days, whereas the TMEO-modified alumina wet gel (A-T0.8) shows little shrinkage (∼5%) and no cracking. It should be noted that the transparency of the wet gel becomes lower after TMEO modification. The appearances of the unmodified and TMEO-modified alumina aerogels after drying and heat treatment are shown in Fig. 2. After drying, the cracks of the unmodified alumina wet gel become larger while there is no cracking for TMEO-modified alumina aerogel. The effect of the amount of TMEO on appearance and gelation time during sol–gel process is also studied. It is found that, when the molar ratio of TMEO to alumina is below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8, it shows longer gelation time and less shrinkage and crack during aging and drying with the increase of the molar ratio of TMEO to alumina. When the molar ratio of TMEO to alumina is in range 1
2.8, it shows longer gelation time and less shrinkage and crack during aging and drying with the increase of the molar ratio of TMEO to alumina. When the molar ratio of TMEO to alumina is in range 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1–1
2.1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8, the gelation time is further increased and only soft wet gels are formed. When the amount of TMEO is too high (TMEO/Al > 1
2.8, the gelation time is further increased and only soft wet gels are formed. When the amount of TMEO is too high (TMEO/Al > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1), no gelation occurs. During sol–gel process, the molar ratios of TMEO to alumina are kept at four different values: 1
2.1), no gelation occurs. During sol–gel process, the molar ratios of TMEO to alumina are kept at four different values: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.8, 1
6.8, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7, 1
4.7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8, 1
3.8, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8. It is found that the aerogel shrinks and cracks little during aging and high-temperature SCFD when the molar ratio of TMEO to alumina is 1
2.8. It is found that the aerogel shrinks and cracks little during aging and high-temperature SCFD when the molar ratio of TMEO to alumina is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8.
3.8.
 | ||
| Fig. 2 Photograph of the aerogels before and after heat treatment at 1200 °C: (a) sample A, (b) sample A-T0.8, (c) sample A-T0.8–A/T. | ||
3.2. The effect of TMEO modification on microstructure
The hydroxyl groups on aerogel surface could react with many groups such as hydroxyl groups, alkoxy groups, isocyanate.27–33 This makes it possible to be modified by reagents with these groups. During the sol–gel process of TMEO-modified alumina wet gel synthesis, TMEO is expected to react with the hydroxyl groups on the alumina surface to form methyl siloxyl groups on the alumina surface which reduces the amount of –OH and produces –Si–(CH3)3 on the alumina surface. Hence, the condensation of the neighbouring –OH on the alumina surface would be inhibited during aging and drying, which effectively restricts the crack and shrinkage of alumina wet gels during aging and drying. Fig. 3 shows the FTIR spectra of the unmodified and TMEO-modified alumina aerogels. As is shown in Fig. 3, the peaks at 2904 cm−1, 2927 cm−1 and 2972 cm−1 are assigned to C–H bond. The peaks at 1124 cm−1 and 1076 cm−1 are attributed to the δas Al–O–H and δs Al–O–H modes of boehmite and the bands at 783 cm−1, 619 cm−1 and 486 cm−1 are attributed to the Al–O mode of boehmite.34,35 It is worth noting that the TMEO-modified alumina aerogels show stronger peaks of C–H compared to the unmodified one, which indicates that TMEO has reacted with the surface hydroxyl groups and be linked to the alumina surfaces.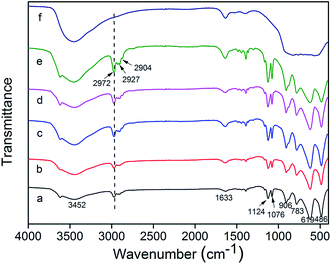 | ||
| Fig. 3 FTIR spectra of unmodified and TMEO-modified alumina aerogels: (a) A, (b) A-T0.45, (c) A-T0.65, (d) A-T0.8, (e) A-T1.1, (f) A-T0.8-1000 °C. | ||
From FTIR (Fig. 3) we also can see that the peaks of C–H become stronger with the increase of the amount of TMEO during sol–gel process. This indicates that more C–H is introduced into the alumina aerogel with more TMEO added. However, after heat treatment at 1000 °C, the peaks of C–H vanish, which indicates that the –CH3 is decomposed after heat treatment at high temperatures. Meanwhile, the bands at 1124 cm−1, 1076 cm−1, 906 cm−1, 783 cm−1, 619 cm−1 and 486 cm−1 correspond to boehmite,36 and vanish after heat treatment at 1000 °C, which is attributed to the phase transition. The phase transition is confirmed by the XRD analysis below. During SCFD, the modifiers TMEO and partially hydrolyzed ASB would react with the –OH on the alumina surface, and thus alumina as well as silica are deposited onto the alumina surface, which results in conformal alumina–silica coatings on the alumina nanoparticles. Scheme 1 shows the schematic representation of TMEO modification mechanism during sol–gel and SCFD process.
The morphology of the unmodified and TMEO-modified alumina aerogels is shown in the TEM images (Fig. 4). We can see that both the samples A and A-T0.8 exhibit randomly interconnected networks made up of leaf-like nanoparticles with thickness of 1–3 nm and length of 20–90 nm. However, after heat treatment at 1200 °C, the morphologies of samples A and A-T0.8 are quite different. The sample A is composed of much thicker rod-like particles with thickness of 6–20 nm and length of 40–120 nm, whereas sample A-T0.8 retains most of the leaf-like particles with thickness of 5–11 nm and length of 35–100 nm and shows some irregular spherical particles. This indicates that alumina particle growth upon heat treatment is effectively restricted after TMEO modification. Both the as-prepared unmodified and TMEO-modified alumina aerogels do now show distinct reflections or diffraction rings as shown in their electron diffractions (the inset in Fig. 4a and b), which indicates the low degree of crystallinity. Nevertheless, the degree of crystallinity of the TMEO-modified alumina aerogel is much lower than that of the unmodified one after heat treatment at 1200 °C (see inset in Fig. 4a and b). The morphology of the alumina aerogel with ASB/TMEO modification during SCFD (the sample A-T0.8–A/T) is also studied through TEM images. It can be seen that the as-prepared sample A-T0.8–A/T shows larger leaf-like particles with thickness of 10–20 nm and length of 70–220 nm. After heat treatment at 1200 °C, it still retains the leaf-like particles. From the stronger electron diffraction spot (inset in Fig. 4c) we can see that the as-prepared alumina aerogel with modification during SCFD (sample A-T0.8–A/T) shows higher degree of crystallinity compared to those without modification during SCFD.
 | ||
| Fig. 4 TEM images of the aerogels before and after heat treatment at 1200 °C: (a) sample A, (b) sample A-T0.8, (c) sample A-T0.8–A/T. | ||
3.3. The effect of TMEO modification on heat resistance
It is found that TMEO modification effectively improves the heat resistance of alumina aerogels. When the molar ratio of TMEO to alumina is below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 during sol–gel process, the more TMEO added the less the alumina aerogel shrinks after heat treatment above 1000 °C. As is shown in Table 2, the unmodified alumina aerogel (sample A) shrinks as high as 12% and 45% after heat treatment at 1000 °C and 1200 °C respectively, whereas the TMEO-modified alumina aerogel with molar ratio of TMEO to alumina of 1
2.8 during sol–gel process, the more TMEO added the less the alumina aerogel shrinks after heat treatment above 1000 °C. As is shown in Table 2, the unmodified alumina aerogel (sample A) shrinks as high as 12% and 45% after heat treatment at 1000 °C and 1200 °C respectively, whereas the TMEO-modified alumina aerogel with molar ratio of TMEO to alumina of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8 (sample A-T0.8) shrinks only 5% and 19% after heat treatment at 1000 °C and 1200 °C respectively. From Fig. 2 we can also clearly see that the shrinkage of alumina aerogel after heat treatment at 1200 °C is significantly reduced by TMEO modification. The less shrinkage is attributed to the introduction of silica on the alumina surface by TMEO modification. During heat treatment at high temperatures, the alumina particles are pulled together through condensation of neighboring –OH, which makes these particles grow much larger than the original ones. This may lead to a series of phase transitions, decrease of surface area and sintering of the alumina aerogel.26 After TMEO modification, the –Si–(CH3)3 on the alumina surface could produce small silica particles that restrict advancing of grain boundaries at high temperatures, which may effectively restrict the crystal growth upon heat treatment.37 The shrinkage is thus effectively reduced after heat treatment. After ASB/TMEO modification during SCFD, the shrinkage of alumina aerogel is further reduced (there is no visible shrinkage for sample A-T0.8–A/T after heat treatment at 1200 °C), which confirms that the heat resistance is further enhanced by ASB/TMEO modification during SCFD. This is because more boehmite crystals are produced by introducing of modifier ASB during SCFD and crystal growth upon heat treatment is further restricted by introducing of TMEO during SCFD. More boehmite crystals enhance the strength of the alumina nanoparticles and thus lower their cracking upon heat treatment, which enhances the heat resistance accordingly. The boehmite crystals are confirms by the XRD analysis below. In addition, we found that the alumina aerogel shows the best heat resistance when the molar ratio of modifier TMEO to ASB is 1
3.8 (sample A-T0.8) shrinks only 5% and 19% after heat treatment at 1000 °C and 1200 °C respectively. From Fig. 2 we can also clearly see that the shrinkage of alumina aerogel after heat treatment at 1200 °C is significantly reduced by TMEO modification. The less shrinkage is attributed to the introduction of silica on the alumina surface by TMEO modification. During heat treatment at high temperatures, the alumina particles are pulled together through condensation of neighboring –OH, which makes these particles grow much larger than the original ones. This may lead to a series of phase transitions, decrease of surface area and sintering of the alumina aerogel.26 After TMEO modification, the –Si–(CH3)3 on the alumina surface could produce small silica particles that restrict advancing of grain boundaries at high temperatures, which may effectively restrict the crystal growth upon heat treatment.37 The shrinkage is thus effectively reduced after heat treatment. After ASB/TMEO modification during SCFD, the shrinkage of alumina aerogel is further reduced (there is no visible shrinkage for sample A-T0.8–A/T after heat treatment at 1200 °C), which confirms that the heat resistance is further enhanced by ASB/TMEO modification during SCFD. This is because more boehmite crystals are produced by introducing of modifier ASB during SCFD and crystal growth upon heat treatment is further restricted by introducing of TMEO during SCFD. More boehmite crystals enhance the strength of the alumina nanoparticles and thus lower their cracking upon heat treatment, which enhances the heat resistance accordingly. The boehmite crystals are confirms by the XRD analysis below. In addition, we found that the alumina aerogel shows the best heat resistance when the molar ratio of modifier TMEO to ASB is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8 during SCFD. Therefore, we have maintained the molar ratio of modifier TMEO to ASB at this value for all the samples during SCFD.
3.8 during SCFD. Therefore, we have maintained the molar ratio of modifier TMEO to ASB at this value for all the samples during SCFD.
| Sample | Density (mg cm−3) | Surface area (m2 g−1) | Pore volume (cm3 g−1) | ΔL/L0a (%) | |||
|---|---|---|---|---|---|---|---|
| 300 °C | 1200 °C | 300 °C | 1200 °C | 1000 °C | 1200 °C | ||
| a Shrinkage after heat treatment. L0 is the original diameter of the sample and ΔL is diameter decrement. | |||||||
| A | 93 | 504 | 84 | 3.20 | 0.16 | 12 | 45 |
| A-T0.45 | 88 | 514 | 103 | 3.23 | 0.20 | 9 | 31 |
| A-T0.8 | 91 | 512 | 118 | 3.27 | 0.25 | 5 | 19 |
| A–A/T | 135 | 214 | 141 | 1.08 | 0.29 | 2 | 5 |
| A-T0.8–A/T | 129 | 237 | 147 | 1.13 | 0.33 | 0 | 0 |
The specific surface areas after heat treatment at high temperatures are also enhanced through TMEO modification. Although the specific surface area of the as-prepared alumina aerogel shows little change, the specific surface area at 1200 °C is increased from 84 m2 g−1 (sample A) to 118 m2 g−1 (sample A-T0.8) after TMEO modification during sol–gel process. If the alumina aerogel is further modified by ASB and TMEO during SCFD, the specific surface area at 1200 °C can be enhanced up to 147 m2 g−1 (sample A-T0.8–A/T). Fig. 5 shows the N2 adsorption/desorption isotherms and pore size distributions of samples A and A-T0.8–A/T before and after heat treatment at 1200 °C. It can be seen from the isotherms (Fig. 5a and b) that both the samples A and A-T0.8–A/T show type IV isotherms, indicating that they are typical mesoporous materials.38–43 As is shown in Fig. 5c and d, compared to unmodified sample A, A-T0.8–A/T exhibits smaller variation of pore size distribution before and after heat treatment at 1200 °C. Table 2 and Fig. 5c and d also show that A-T0.8–A/T exhibits much larger pore volume (0.33 cm3 g−1) than that of A (0.16 cm3 g−1) after heat treatment at 1200 °C. This confirms that the heat resistance of alumina aerogels is significantly enhanced by TMEO modification.
Fig. 6 shows the XRD patterns for typical alumina aerogels. All the as-prepared aerogels including unmodified and modified samples are composed of boehmite AlO(OH).44–46 The difference is that the peaks of boehmite become weaker after TMEO modification during sol–gel process and become stronger after further modification during SCFD. The weaker peaks of boehmite after TMEO modification during sol–gel process are because the methyl siloxyl groups on the alumina surface restrict the crystal growth during SCFD. The stronger peaks of boehmite after ASB/TMEO modification during SCFD are because more boehmite crystals are produced by introducing of ASB during SCFD. After heat treatment at 1000 °C, the unmodified alumina aerogel is changed to θ-Al2O3, whereas TMEO-modified one is δ-Al2O3.47 After heat treatment at 1200 °C, θ-Al2O3 emerges for both unmodified and modified samples.48 However, the TMEO-modified alumina aerogel shows much weaker peaks of θ-Al2O3 compared to the unmodified one, indicating the lower degree of crystallinity of the TMEO-modified alumina aerogel compared to the unmodified one after heat treatment at 1200 °C. The phase transitions are consistent with the TEM and FTIR analysis above. Both XRD and TEM analysis confirm that crystal growth upon heat treatment is effectively restricted after TMEO modification.
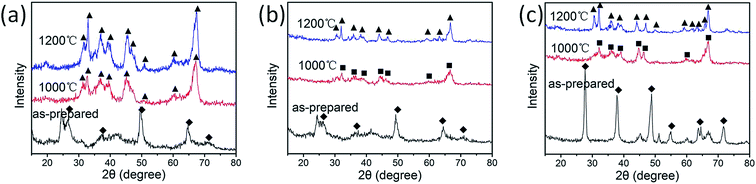 | ||
| Fig. 6 XRD patterns for typical aerogels before and after heat treatment at high temperatures: (a) sample A, (b) sample A-T0.8, (c) sample A-T0.8–A/T. (◆) Boehmite, (■) δ-Al2O3, (▲) θ-Al2O3. | ||
3.4. The effect of TMEO modification on adsorption and high-temperature thermal insulation performance
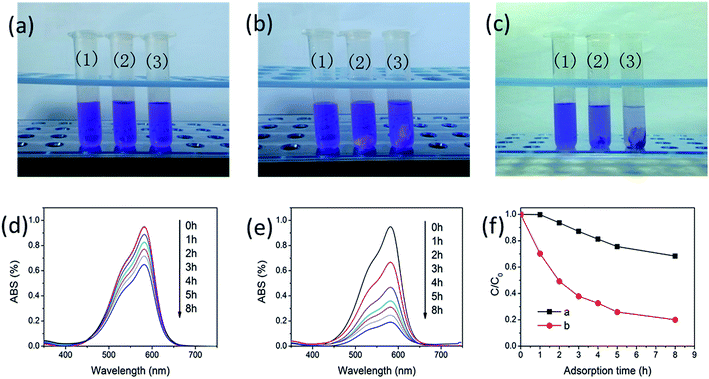
High thermal stability, high surface area at high temperatures make TMEO-modified alumina aerogel exhibit enhanced performance such as high-temperature thermal insulation and adsorption properties. Higher surface area and pore volume is presumed to own better adsorption performance for dye. Thus, gentian violet adsorption experiment is performed to confirm the enhancement of specific surface area and pore volume of alumina aerogels at high temperatures after TMEO modification. The adsorption property of the TMEO-modified alumina aerogel for gentian violet after heat treatment at 1200 °C is tested as shown in Fig. 7. The unmodified alumina aerogel (sample A) and TMEO-modified alumina aerogel (sample A-T0.8–A/T) with the equal weight are immersed in two gentian violet solutions with the same concentration (0.026 mg mL−1) respectively. Another gentian violet solution without aerogel is for comparison. The absorption spectra of gentian violet solution in the presence of aerogels at various adsorption treatment times are measured by a UV/VIS/NIR Spectrophotometer. After adsorption for 8 h, the concentration percentage of gentian violet with unmodified alumina aerogel remains 68.35%, whereas the concentration percentage of gentian violet with TMEO-modified alumina aerogel is as low as 20%. From the photographs of gentian violet before and after adsorption (Fig. 7a–c) we can also see the better adsorption performance of the TMEO-modified alumina aerogel for gentian violet after heat treatment at 1200 °C. The gentian violet solution with the unmodified alumina aerogel remains purple while the gentian violet solution with the TMEO-modified alumina aerogel becomes nearly transparent after adsorption for 8 h.The TMEO modified alumina aerogel also exhibits excellent high-temperature thermal insulation performance. As is shown in Table 3, the thermal conductivities of the unmodified alumina aerogel (sample A) at 800 and 1000 °C is as high as 0.33 and 0.47 W (m−1 K−1) respectively while those of the TMEO-modified alumina aerogel (sample A-T0.8–A/T) are as low as 0.13 and 0.18 W (m−1 K−1) respectively. These values are lower than those of the reported alumina aerogels.4,13 The enhanced high-temperature thermal insulation performance of the TMEO-modified alumina aerogel is attributed to its improved heat resistance, small pore size (mainly 1–60 nm for A-T0.8–A/T), high pore volume (0.33 cm3 g−1 for A-T0.8–A/T) and high surface area at 1200 °C. High pore volume decreases solid-phase thermal conduction and smaller pore size leads to lower gas-phase conduction, because the kinetic energy exchange of the gas molecules inside the porous body becomes more inefficient, which leads to low thermal conductivities at high temperatures. The super heat-resistant, high-surface-area, high pore volume TMEO-modified alumina aerogel could find its high-temperature applications in catalysts, thermal insulations, adsorptions, etc.
4. Conclusions
Super heat-resistant TMEO-modified alumina aerogels are prepared via a sol–gel method through TMEO modification during sol–gel and SCFD process. It is found that when the molar ratio of TMEO to alumina is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8 during sol–gel process and SCFD process, the aerogel shows the best heat resistance. It has no crack and little shrinkage during aging and drying, and shows no shrinkage and high specific surface area of 147 m2 g−1 after heat treatment at 1200 °C. The enhanced heat resistance is attributed to the introduction of silica on alumina surface through TMEO modification. After TMEO modification, the condensation of the neighbouring –OH on the alumina surface is restricted and thus the crack and shrinkage of alumina wet gels during aging and drying is effectively inhibited. In addition, crystal growth upon heat treatment is restricted by introduction of –Si–(CH3)3 that can produce small particles that restrict growth of grain boundaries at high temperatures, which leads to the enhanced heat resistance. The TMEO-modified alumina aerogel remains excellent adsorption performance for gentian violet after fired at 1200 °C and exhibits ultra-low thermal conductivities at high temperatures owing to its high thermal stability. It could have future high-temperature application such as thermal insulations, catalysts and adsorption, etc.
3.8 during sol–gel process and SCFD process, the aerogel shows the best heat resistance. It has no crack and little shrinkage during aging and drying, and shows no shrinkage and high specific surface area of 147 m2 g−1 after heat treatment at 1200 °C. The enhanced heat resistance is attributed to the introduction of silica on alumina surface through TMEO modification. After TMEO modification, the condensation of the neighbouring –OH on the alumina surface is restricted and thus the crack and shrinkage of alumina wet gels during aging and drying is effectively inhibited. In addition, crystal growth upon heat treatment is restricted by introduction of –Si–(CH3)3 that can produce small particles that restrict growth of grain boundaries at high temperatures, which leads to the enhanced heat resistance. The TMEO-modified alumina aerogel remains excellent adsorption performance for gentian violet after fired at 1200 °C and exhibits ultra-low thermal conductivities at high temperatures owing to its high thermal stability. It could have future high-temperature application such as thermal insulations, catalysts and adsorption, etc.
Acknowledgements
This research is financially supported by National Natural Science Foundation of China (U1230113), National key Technology R&D Program of China (2013BAJ01B01) and Shanghai Committee of Science and Technology (11nm0501600, 12nm0503001). We also thank the support from Bayer-Tongji Eco-Construction & Material Academy.References
- N. Husing and U. Schubert, Angew. Chem., Int. Ed., 1998, 37, 22 CrossRef CAS.
- A. S. Dorcheh and M. H. Abbasi, J. Mater. Process. Technol., 2008, 199, 10 CrossRef PubMed.
- J. Fricke and A. Emmerling, Struct. Bonding, 1992, 77, 37 CrossRef CAS.
- J. F. Poco, J. H. Satcher and L. W. Hrubesh, J. Non-Cryst. Solids, 2001, 285, 57 CrossRef CAS.
- A. Pierre, R. Begag and G. Pajonk, J. Mater. Sci., 1999, 34, 4937 CrossRef CAS.
- L. Wu, Y. Huang, Z. Wang, L. Liu and H. Xu, Appl. Surf. Sci., 2010, 256, 5973 CrossRef CAS PubMed.
- T. F. Baumann, A. E. Gash, S. C. Chinn, A. M. Sawvel, R. S. Maxwell and J. H. Satcher, Chem. Mater., 2005, 17, 395 CrossRef CAS.
- J. B. Miller and E. I. Ko, Catal. Today, 1998, 43, 51 CrossRef CAS.
- A. A. Khaleel and K. J. Klabunde, Chem.–Eur. J., 2002, 8, 3991 CrossRef CAS.
- L. L. Bihan, F. Dumeignil, E. Payen and J. Grimblot, J. Sol-Gel Sci. Technol., 2002, 24, 113 CrossRef.
- A. C. Pierre, E. Elaloui and G. M. Pajonk, Langmuir, 1998, 14, 66 CrossRef CAS.
- Y. Tokudome, K. Nakanishi, K. Kanamori and K. Fujita, J. Colloid Interface Sci., 2009, 338, 506 CrossRef CAS PubMed.
- G. Zu, J. Shen, X. Wei, X. Ni, Z. Zhang, J. Wang and G. Liu, J. Non-Cryst. Solids, 2011, 357, 2903 CrossRef CAS PubMed.
- D. J. Suh, T. J. Park, J. H. Kim and K. L. Kim, Chem. Mater., 1997, 9, 1905 Search PubMed.
- R. Sui and P. Charpentier, Chem. Rev., 2012, 112, 3057 CrossRef CAS PubMed.
- L. Ji, J. Lin, K. L. Tan and H. C. Zeng, Chem. Mater., 2000, 12, 931 CrossRef CAS.
- J. Walendziewski and M. Stolarski, React. Kinet. Catal. Lett., 2000, 71, 201 CrossRef CAS.
- M. S. Bono Jr and A. M. Anderson, J. Sol-Gel Sci. Technol., 2010, 53, 216 CrossRef.
- B. Himmel, T. Gerber, H. Burger, G. Holzhuter and A. Olbertz, J. Non-Cryst. Solids, 1995, 186, 149 CrossRef CAS.
- S. Keysar, G. E. Shter, Y. Dehazan, Y. Cohen and G. S. Grader, Chem. Mater., 1997, 9, 2464 CrossRef CAS.
- Y. Mizushima and M. Hori, J. Mater. Res., 1993, 8, 2993 CrossRef CAS.
- R. Saliger, T. Heinrich, T. Gleissner and J. Fricke, J. Non-Cryst. Solids, 1995, 186, 113 CrossRef CAS.
- T. Horiuchi, T. Osaki, T. Sugiyama, K. Suzuki and T. Mori, J. Non-Cryst. Solids, 2001, 291, 187 CrossRef CAS.
- P. R. Aravind, P. Shajesh, S. Smitha, P. Mukundan and K. G. Warrier, J. Am. Ceram. Soc., 2008, 91, 1326 CrossRef CAS PubMed.
- T. Osaki, K. Nagashima, K. Watari and K. Tajiri, J. Non-Cryst. Solids, 2007, 353, 2436 CrossRef CAS PubMed.
- G. Zu, J. Shen, L. Zou, W. Wang, Y. Lian, Z. Zhang and A. Du, Chem. Mater., 2013, 25, 4757 CrossRef CAS.
- N. Leventis, Acc. Chem. Res., 2007, 40, 874 CrossRef CAS PubMed.
- N. Leventis and H. Lu, Polymer-Crosslinked Aerogels, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer, New York, 2011, pp. 251–285 Search PubMed.
- D. P. Mohite, Z. J. Larimore, H. Lu, J. T. Mang, C. Sotiriou-Leventis and N. Leventis, Chem. Mater., 2012, 24, 3434 CrossRef CAS.
- N. Leventis, C. S. Leventis, G. Zhang and A. M. M. Rawashdeh, Nano Lett., 2002, 2, 957 CrossRef CAS.
- Z. Wang, Z. Dai, J. Wu, N. Zhao and J. Xu, Adv. Mater., 2013, 25, 1 CrossRef.
- J. P. Randall, M. A. B. Meador and S. C. Jana, ACS Appl. Mater. Interfaces, 2011, 3, 613 CAS.
- D. Sanli and C. Erkey, ACS Appl. Mater. Interfaces, 2013, 5, 11708 CAS.
- H. Hou, Y. Xie, Q. Yang, Q. Guo and C. Tan, Nanotechnology, 2005, 16, 741 CrossRef CAS.
- D. Y. Li, Y. S. Lin, Y. C. Li, D. L. Shieh and J. L. Lin, Microporous Mesoporous Mater., 2008, 108, 276 CrossRef CAS PubMed.
- T. Z. Ren, Z. Y. Yuan and B. L. Su, Langmuir, 2004, 20, 1531 CrossRef CAS.
- N. L. Wu, S. Y. Wang and I. A. Rusakova, Science, 1999, 285, 1375 CrossRef CAS.
- Y. Kong, G. Jiang, M. Fan, X. Shen, S. Cui and A. G. Russell, Chem. Commun., 2014, 50, 12158 RSC.
- Y. Kong, X.-D. Shen and S. Cui, Mater. Lett., 2014, 117, 192 CrossRef CAS PubMed.
- Y. Kong, Y. Zhong, X. Shen, S. Cui and M. Fan, Microporous Mesoporous Mater., 2014, 197, 77 CrossRef CAS PubMed.
- Y. Kong, Y. Zhong, X. Shen, S. Cui, M. Yang, K. Teng and J. Zhang, J. Non-Cryst. Solids, 2012, 358, 3150 CrossRef CAS PubMed.
- Y. Kong, X. Shen, S. Cui and M. Fan, Ceram. Int., 2014, 40, 8265 CrossRef CAS PubMed.
- Y. Kong, Y. Zhong, X. Shen, L. Cu, S. Cui and M. Yang, Mater. Lett., 2013, 99, 108 CrossRef CAS PubMed.
- A. H. Munhoz Jr, R. W. Novickis, S. B. Faldini, R. R. Ribeiro, C. Y. Maeda and L. F. D. Miranda, Adv. Sci. Technol., 2010, 76, 184 CrossRef.
- J. A. Wang, X. Bokhimi, A. Morales and O. Novaro, J. Phys. Chem. B, 1999, 103, 299 CrossRef CAS.
- A. Boumaza, L. Favaro, J. Ledion, G. Sattonnay, J. B. Brubach, P. Berthet, A. M. Huntz, P. Roy and R. Tetot, J. Solid State Chem., 2009, 182, 1171 CrossRef CAS PubMed.
- P. S. Santos, H. S. Santos and S. P. Toledo, Mater. Res., 2000, 3, 104 CAS.
- M. Bodaghi, A. R. Mirhabibi, H. Zolfonun, M. Tahriri and M. Karimi, Phase Transitions, 2008, 81, 571 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |