Li(glycine)(CF3SO3) as an effective and recoverable catalyst for the preparation of 3,4-dihydropyrimidine-2-(1H)-one under solvent-free conditions†
Esmayeel Abbaspour-Gilandeh*a,
Seyyedeh Cobra Azimib and
Aidin Mohammadi-Barkchaic
aYoung Researchers and Elites Club, Ardabil Branch, Islamic Azad University, Ardabil, Iran. E-mail: abbaspour1365@yahoo.com; Fax: +98 4513332238
bYoung Researchers and Elites Club, Rasht Branch, Islamic Azad University, Rasht, Iran
cDepartment of Applied Chemistry, Ardabil Branch, Islamic Azad University, Ardabil, Iran
First published on 17th October 2014
Abstract
An efficient solvent-free protocol for the synthesis of 3,4-dihydropyrimidine-2-(1H)-one by the one-pot condensation of aldehyde, ethyl acetoacetate and urea using Li(glycine)(CF3SO3) as a reusable acidic ionic liquid is reported. These reactions have the advantages in this work of clean reaction, simple purification, short reaction time and high yields.
Introduction
Ionic liquids (ILs) are synthetic salts that have melting points below 100 °C. In these compounds, one or both of the ions are organic species. Delocalized charge of one of the ions prevents formation of a stable crystal lattice and strong electrostatic forces hold the ions together. The poor coordination of the ions makes these compounds are liquid below 100 °C or at room temperature.1 In recent years, using of ionic liquids in scientific researches have been surged considerably.2,3 This growth exactly is associated with their unique properties such as negligible vapor pressure, low toxicity and high thermal and chemical stabilities. For this reason, ILs have been used as organic solvents in applications, such as catalysis and synthesis.4 Moreover, a series of ILs have proven to be suitable solvents for enzyme reactions.5 High separation ability, easy operation and zero contamination of the distilled products are other advantages for ILs, and have attractive characteristics for applications in industrial processes and employed as an agent of mass separation azeotropic mixtures or close boiling points mixtures.6,7For the synthesis of complex and novel molecular structures, multicomponent reactions (MCRs) are powerful and popular tools.8–11 This reactions are the major advantages such as shorter reaction time, energy saving, high atom economy and lower cost.12–14 MCRs provide synthetic access to large compound libraries and do not need deprotection and protection steps. These reactions also are much more environmentally friendly.15–18
Dihydropyrimidinones (DHPMs) with their unique characteristics such as antibacterial, antioxidant, anti HIV and anticancer have widely spectrum for biological activities19 and used for synthesis of heterocycle compounds.20 Previously different derivatives of 3,4-dihydropyrimidine-2-(1H)-one have exhibited calcium channel modulators and neuropeptide Y (NPY) antagonist.21 In 1893, an Italian chemist, Pietro Biginelli reported a simple and direct reaction for production of DHPMs. This method is based on one-pot condensation of an aldehyde, a β-ketoester and a urea that proceed under strongly acidic conditions.22 But this protocol suffers harsh condition, long reaction time and low yield,23 especially in the case of hetero aromatic aldehydes.24 Recently, several elegant multicomponent strategies for the synthesis of 3,4-dihydropyrimidine-2-(1H)-one by multicomponent reactions utilizing catalysts have been reported.25–39 However some of these methods suffer from disadvantages such as low yield,37 prolonged reaction time,34 use of excess of catalysts,30 use of toxic organic solvents31 and harsh reaction conditions.27 Due to their wide range of applications, these compounds have received a great deal of attention in connection with their synthesis.
Experimental
General
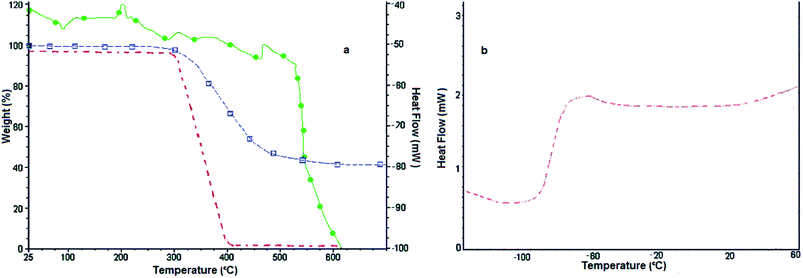
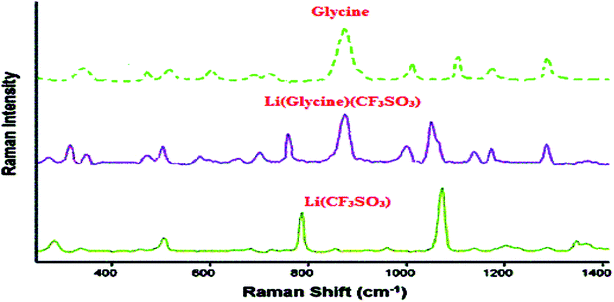
All chemical materials were purchased from Fluka and Merck companies and used without further purification. The purity determination of the product and reaction monitoring were accomplished by TLC on silica gel PolyGram SILG/UV 254 plates. The melting points were determined using an Electrothermal 9100 apparatus and are uncorrected. The IR spectra were recorded as KBr pellets on a Shimadzu Corporation 200-91-527 instrument. The NMR spectra were obtained using a BRUKER DRX-300 AVANCE spectrometer at room temperature in CDCl3 using TMS as an internal standard. Inductively coupled plasma optical emission spectrometry (ICP-OES), measurements were performed on an ICP Varian 735-ES. Raman data were acquired on a Thermo DXR with a 633 nm laser. Thermo gravimetric analyses (TGA) were conducted by using a TGA PYRIS 1 thermoanalyzer instrument. Samples were heated from 25 to 600 °C at ramp 10 °C min−1 under N2 atmosphere and a TA Instruments DSC 2010 (with a quench cooling accessory, N2 flow) with 10 °C min−1 heating.Catalyst preparation
General procedure for preparation of 3,4-dihydropyrimidine-2-(1H)-one derivative
A mixture of aldehyde (1 mmol), ethyl acetoacetate (1 mmol), urea (2 mmol) and Li(glycine)(CF3SO3) (10 mol%) was sealed and stirred at 80 °C under solvent-free conditions. After completion of the reaction, as indicated by precipitation of solid products from the liquid reaction mixture and TLC experiments, the reaction mixture was diluted with ethanol (10 mL) and stirred for 5 min. The solid catalyst was successfully recovered after evaporation of water under reduced pressure which can be reused. The product was found to be pure and no further purification was necessary.Spectral data of the products
Results and discussion
Catalyst characterization
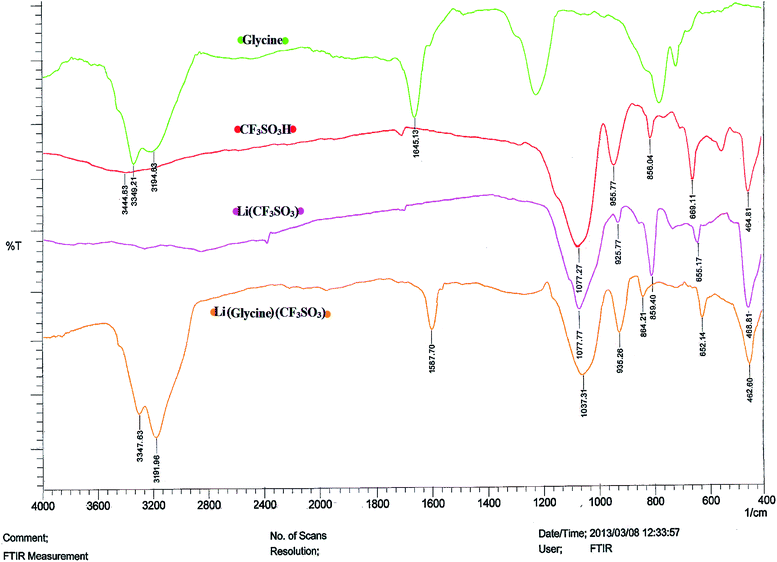
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group. Also, appearing bands at 699 cm−1 and 955 cm−1 to be assigned to the symmetric and asymmetric (S–O) group. The spectrum shows a relatively broad band in the range of 3000 to 3600 cm−1 which indicates the presence of SO3H group. In the case of Li(CF3SO3), the peaks at about 655 cm−1 and 925 cm−1 are due to the Li+ ion which is associated with a decrease in the ν(S–O) vibration mode. Also, appearing band in 1077 cm−1 correspond to the S
O) group. Also, appearing bands at 699 cm−1 and 955 cm−1 to be assigned to the symmetric and asymmetric (S–O) group. The spectrum shows a relatively broad band in the range of 3000 to 3600 cm−1 which indicates the presence of SO3H group. In the case of Li(CF3SO3), the peaks at about 655 cm−1 and 925 cm−1 are due to the Li+ ion which is associated with a decrease in the ν(S–O) vibration mode. Also, appearing band in 1077 cm−1 correspond to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations. Absenting band around 3000 to 3600 cm−1 can be attributed to the replacement of the hydrogen atom by the Li+ ion on the (S–O) group. From these data can be inferred that the novel ionic liquid has been confirmed. Significant point in the spectrum of glycine is the presence of COO− band at 1645 cm−1 and NH bands in the range between 3100–3600 cm−1 which contributed to identification of the ionic liquid. The IR spectra of ionic liquid Li(glycine)(CF3SO3) refer to the successful formation of this compound. All bands ranging from bands of the glycine and CF3SO3 have been appeared in their place. In this spectra, the band of COO− in comparison with the glycine spectra is drawn to the lower absorption and implies that the lithium atom is also attached to the COO− group.
O stretching vibrations. Absenting band around 3000 to 3600 cm−1 can be attributed to the replacement of the hydrogen atom by the Li+ ion on the (S–O) group. From these data can be inferred that the novel ionic liquid has been confirmed. Significant point in the spectrum of glycine is the presence of COO− band at 1645 cm−1 and NH bands in the range between 3100–3600 cm−1 which contributed to identification of the ionic liquid. The IR spectra of ionic liquid Li(glycine)(CF3SO3) refer to the successful formation of this compound. All bands ranging from bands of the glycine and CF3SO3 have been appeared in their place. In this spectra, the band of COO− in comparison with the glycine spectra is drawn to the lower absorption and implies that the lithium atom is also attached to the COO− group.
In continuation of our studies on the synthesis of various bioactive compounds,41 we decided to investigate the synthesis of 3,4-dihydropyrimidine-2-(1H)-one derivatives in the presence of Li(glycine)(CF3SO3) as a novel catalyst under solvent-free conditions. The reported route is an appropriate, efficacious and novel method for condensation of aldehyde, ethyl acetoacetate and urea in the presence of Li(glycine)(CF3SO3) as an acidic ionic liquid (Scheme 1).
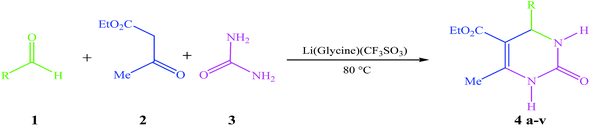 | ||
| Scheme 1 One-pot three-component reaction of different aldehydes, ethyl acetoacetate and urea catalyzed by Li(glycine)(CF3SO3) ionic liquid. | ||
First, the optimization of the reaction temperature and the amount of the catalyst required for the reaction of aldehyde (1 mmol), ethyl acetoacetate (1 mmol) and urea (2 mmol) was standardized by carrying out the reaction in the presence of different amount of Li(glycine)(CF3SO3) at different temperature ranging 25 °C to 90 °C for different periods of time by conventional heating (Table 2). The obtained results show that below 10 mol% of Li(glycine)(CF3SO3), the yield of product was low (Table 2, entry 4), however, increasing in the amount of catalyst had no effect on the yield and only reaction time was prolonged (Table 2, entry 10). Also, increasing or decreasing in temperature of the reaction have a reverse effect on the compound yield. Only at 60 °C and with 20 minutes of reaction time, the highest product yield of 83% was observed (Table 2, entry 7). With consideration to Table 1, the reaction using 10 mol% of catalyst at 80 °C proceeded in highest yield (Table 2, entry 8). Monitoring by TLC has shown detectable progress of the reaction for the acidic ionic liquids such as [HMIm]HSO4 and [BMIm]HSO4, but these ionic liquids were not as effective as Li(glycine)(CF3SO3). The model reaction gave low yield in even long time when it was run in the ionic liquid [BMIm]BF4. Adding 20 mol% of the p-TSA to the [BMIm]BF4 lead to increasing the catalytic activity and rate of the reaction (Table 2, entry 3). Interestingly, when the trial reaction was performed in the absence of the novel IL under same reaction condition, nearly no product detected even after 3 h (Table 2, entry 11), demonstrating, the catalytic role of Li(glycine)(CF3SO3) in the synthesis of desired 3,4-dihydropyrimidine-2-(1H)-one derivatives.
| Entry | Ionic liquid (mol%) | Temperature (°C) | Reaction time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: a mixture of 2-chlorobenzaldehyde (1 mmol), ethyl acetoacetate (1 mmol), urea (2 mmol).b Isolated yield. | ||||
| 1 | [BMIm]HSO4 | 80 | 120 | 81 |
| 2 | [BMIm]BF4 | 80 | 200 | 30 |
| 3 | [BMIm]BF4/p-TSA | 80 | 25 | 60 |
| 4 | Li(glycine)(CF3SO3) (5) | 80 | 30 | 55 |
| 5 | Li(glycine)(CF3SO3) (10) | 25 | 50 | 65 |
| 6 | Li(glycine)(CF3SO3) (10) | 40 | 35 | 72 |
| 7 | Li(glycine)(CF3SO3) (10) | 60 | 20 | 83 |
| 8 | Li(glycine)(CF3SO3) (10) | 80 | 4 | 96 |
| 9 | Li(glycine)(CF3SO3) (10) | 90 | 25 | 75 |
| 10 | Li(glycine)(CF3SO3) (15) | 80 | 20 | 95 |
| 11 | — | 80 | 3 h | Trace |
To improve the yields further and to make the process green, the reaction was run in different solvents (Table 3). It was found that the highest yield (85%) was achieved when the reaction was conducted in PEG as a co-solvent (Table 3, entry 3). Unfortunately, the use of co-solvents system such as DMF, DMSO, CH2Cl2, CHCl3 and toluene resulted in poor to moderate product yields (34–45%) under similar conditions (Table 3, entries 4 to 8). While the reaction proceeded sluggishly in CH3CN (Table 3, entries 9). This solvents plays a negative role by retarding the multi-component pathway. This might be due to the adsorption of solvent on the catalyst surface or the solvent–reactant interactions. Although water and ethanol were proved to be capable of promoting the reaction, in this case, IL destruction and silica chromatography needed to be used in order to separate and purify the product (Table 3, entries 1 and 2). Interestingly, the trial reaction in the presence of IL and in the absence of solvent afforded the desired product 4a under solvent-free condition in high to quantitative yields and less reaction time compared to other referred solvents (Table 3, entry 10).
| Entry | Solvent | Amount of catalyst (mol%) | Temperature (°C) | Reaction time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: a mixture of 2-chlorobenzaldehyde (1 mmol), ethyl acetoacetate (1 mmol), urea (2 mmol) and Li(glycine)(CF3SO3) (1 mL).b Isolated yield. | |||||
| 1 | Water | 10 | 80 | 30 | 25 |
| 2 | EtOH | 10 | 80 | 30 | 20 |
| 3 | PEG | 10 | 80 | 20 | 85 |
| 4 | DMF | 10 | 80 | 30 | 45 |
| 5 | DMSO | 10 | 80 | 20 | 34 |
| 6 | CH2Cl2 | 10 | 80 | 40 | 40 |
| 7 | CHCl3 | 10 | 80 | 55 | 42 |
| 8 | Toluene | 10 | 80 | 52 | 39 |
| 9 | CH3CN | 10 | 80 | 20 | 34 |
| 10 | — | 10 | 80 | 4 | 96 |
Furthermore, a comparative evaluation of the catalytic efficacy of Li(glycine)(CF3SO3) was compared with some of the obtained results in this study with those methodologies which have been reported using other earlier homogeneous and heterogeneous catalysts for the synthesis of 3,4-dihydropyrimidine-2-(1H)-one. It is obvious that a superior methodology in term the use of a renewable catalyst without any post-modification and catalyst loading in most cases has been developed. A demonstrated in Table 4, our method is simpler, efficient, and less time consuming for the synthesis of 5-ethoxycarbonyl-4-phenyl-6-methyl-3,4-dihydro pyrimidin-2(1H)-one.
| Entry | Catalyst and conditions | Reaction time (h) | Yield (%) | References |
|---|---|---|---|---|
| 1 | Silica sulfuric acid/EtOH/heat | 6 | 91 | 42 |
| 2 | Sulfated tungstate/solvent-free/80 °C | 1 | 92 | 43 |
| 3 | BF3·OEt2/CuCl/THF/reflux | 18 | 71 | 28 |
| 4 | Cu(OTf)2/EtOH/100 °C/MW | 1 | 95 | 45 |
| 5 | CD-SO3H/solvent-free/80 °C | 2 | 89 | 46 |
| 6 | PPA-SiO2/CH3CN/reflux | 1 | 88 | 32 |
| 7 | DBSA/water/54 °C | 7 | 89 | 47 |
| 8 | IRMOF-3/solvent-free/reflux | 5 | 89 | 48 |
| 9 | Chiral phosphoric acid/CH2Cl2/25 °C | 4 days | 77 | 56 |
| 10 | Bentonite-/PS-SO3H/solvent-free/120 °C | 35 min | 84 | 57 |
| 11 | Ce(C12H25SO3)/EtOH/80 °C | 8 | 83 | 58 |
| 12 | SnCl2–nano SiO2/EtOH/reflux | 40 min | 92 | 59 |
| 13 | Li(glycine)(CF3SO3)/solvent-free/80 °C | 4 min | 96 | This work |
In order to study the generalize of this procedure, a series of 3,4-dihydropyrimidine-2-(1H)-one derivatives (4a–v) were prepared in excellent yields from different aromatic aldehydes having electron-donating as well as electron-withdrawing groups under the optimal conditions. These results are listed in Table 5. The nature of the functional group on the aromatic ring of the aldehyde exerted a strong influence on the reaction time. It was observed that aromatic aldehydes with both electron-donating and electron-withdrawing groups reacted smoothly to gave the corresponding product in high yield (Table 5, entries 4b–t). Introduction of the electron-donating substituents, such as Me and OMe, caused a decrease of the cytotoxic activity while no significant effect on the cytotoxic activity was observed for the electron-withdrawing substituents such as CN and NO2. Halogen substituted aromatic aldehydes were employed under the reaction conditions excellent yields (92–98%) of the corresponding products (4b–h) were obtained. The ortho and para-substituted gave good results in short time compared to the meta-substituted as there is more steric hindrance for the meta substituted aldehydes such as m-(OMe, –F, –Cl, –Br, –NO2) with the use of Li(glycine)(CF3SO3) catalysts. Insertion of halogens like F, Cl or Br into the aryl group as in (4b–h), induces an increase of the cytotoxicity. These results clearly indicate that the reactions can tolerate a wide range of differently substituted aldehydes.
| Entry | R | Yieldb (%) | Time (min) | Mpc (°C) found | Mp (°C) (Lit.) |
|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (1 mmol), ethyl acetoacetate (1 mmol), urea (2 mmol) and Li(glycine)(CF3SO3) (10 mol%), 80 °C.b Isolated yield.c Melting points are uncorrected. | |||||
| 4a | C6H5– | 97 | 40 | 203–205 | 202–203 (ref. 53) |
| 4b | 2-(Cl)–C6H4– | 98 | 35 | 216–219 | 215–218 (ref. 29) |
| 4c | 3-(Cl)–C6H4– | 95 | 35 | 193–195 | 192–193 (ref. 48) |
| 4d | 4-(Cl)–C6H4– | 97 | 35 | 211–213 | 212–214 (ref. 26) |
| 4e | 3-(Br)–C6H4– | 94 | 30 | 185–187 | 185–186 (ref. 55) |
| 4f | 2,4-(Cl)2–C6H3– | 96 | 30 | 249–251 | 251–252 (ref. 54) |
| 4g | 3,4-(Cl)2–C6H3– | 93 | 35 | 223–225 | 222–223 (ref. 49) |
| 4h | 4-(F)–C6H4– | 94 | 30 | 178–180 | 175–177 (ref. 22) |
| 4i | 2-(NO2)–C6H4– | 95 | 35 | 218–220 | 220–222 (ref. 51) |
| 4j | 3-(NO2)–C6H4 | 93 | 37 | 225–227 | 226–228 (ref. 53) |
| 4k | 4-(NO2)–C6H4– | 97 | 35 | 211–213 | 208–211 (ref. 44) |
| 4l | 2-(OCH3)–C6H4– | 93 | 37 | 258–260 | 259–260 (ref. 42) |
| 4m | 3-(OCH3)–C6H4– | 91 | 37 | 208–210 | 207–208 (ref. 28) |
| 4n | 4-(OCH3)–C6H4– | 92 | 37 | 203–205 | 203–204 (ref. 53) |
| 4o | 3,4-(OCH3)2–C6H3– | 90 | 40 | 176–178 | 174–176 (ref. 49) |
| 4p | 3-(CH3O)–4-(HO)– | 91 | 40 | 231–233 | 233–235 (ref. 53) |
| 4q | 4-(CF3)–C6H4– | 93 | 30 | 177–179 | 173–175 (ref. 50) |
| 4r | 2-(HO)–C6H4– | 91 | 40 | 198–200 | 199–201 (ref. 52) |
| 4s | 4-(HO)–C6H4– | 92 | 40 | 229–231 | 231–233 (ref. 53) |
| 4t | 4-(CH3)–C6H4– | 90 | 40 | 215–216 | 216–217 (ref. 50) |
| 4u | C6H5–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
85 | 40 | 227–229 | 234–236 (ref. 53) |
| 4v | 2-Furyl | 83 | 40 | 204–206 | 205–207 (ref. 53) |
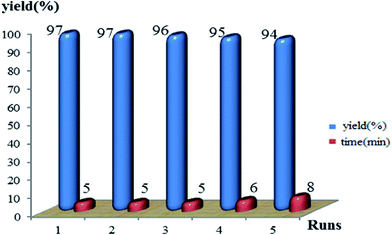
In the next phase of study, the recovery and reuse cycle of Li(glycine) (CF3SO3) was also evaluated. Hence, we investigated the recyclability of Li(glycine)(CF3SO3) for five consecutive cycles to afforded of 5-ethoxycarbonyl-4-phenyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4a). As shown in Fig. 4, this ionic liquid can be recycled at least five times without significant decrease in catalytic activity, the yields ranged from 97% to 94%.
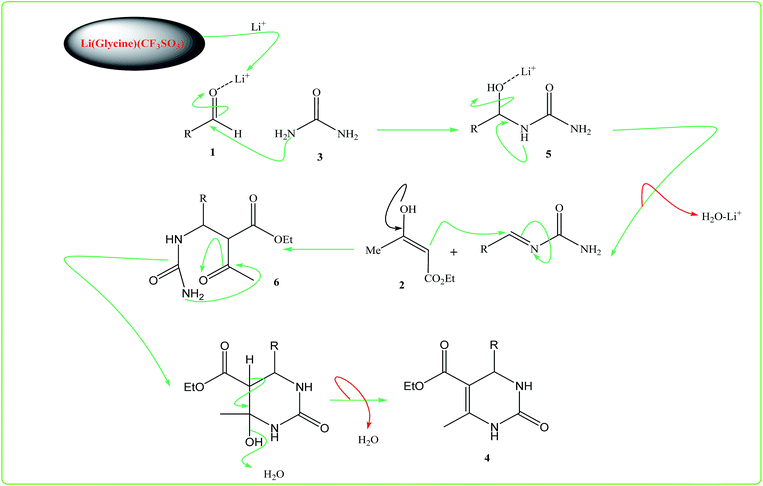
According to these observations, a possible mechanism for the formation of 3,4-dihydro pyrimidin-2(1H)-one is shown in Scheme 2. The reaction occurs via initial formation of the acylimine intermediate (5) by nucleophilic addition of urea (3) to aldehyde (1) followed by dehydration. In this stage, activated 1,3-dicarbonyl compound is attack to the acylimine intermediate and an open-chain ureide (6) be formed which underwent intramolecular cyclization to afford the final product (4).
References
- B. J. Kinzig, P. Sutor, W. G. Sawyer, A. Rennie, P. Dickrell and J. Gresham, ASME World Tribology Congress Proceedings, 2005, WTC2005–63744, p. 509 Search PubMed.
- S. Lee, Chem. Commun., 2006, 1049 RSC.
- N. Jain, N. A. Kumar, S. Chauhan and S. M. S. Chauhan, Tetrahedron, 2005, 61, 1015 CrossRef CAS PubMed.
- K. E. Gutowski, G. A. Broker, H. D. Willauer, J. G. Huddleston, R. P. Swatloski, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2003, 125, 6632 CrossRef CAS PubMed.
- A. P. M. Tavares, O. Rodriguez and E. A. Macedo, Biotechnol. Bioeng., 2008, 101, 201 CrossRef CAS PubMed.
- M. Seiler, C. Jork, A. Kavarnou, W. Arlt and R. Hirsch, AIChE J., 2004, 50, 2439 CrossRef CAS.
- V. H. Alvarez, R. Maciel-Filho, M. Aznar and S. Mattedi, Fenómenos de Transferencia, 2009, 4, 8 Search PubMed.
- For a Recent Monograph, see: Multicomponent Reactions, ed. J. Zhu and H. Bienayme, Wiley-VCH, Weinheim, Germany, 2005 Search PubMed.
- J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 2009, 15, 1300 CrossRef CAS PubMed.
- S. L. Schreiber and M. D. Burke, Angew. Chem., Int. Ed., 2004, 43, 46 CrossRef PubMed.
- P. Arya, D. T. H. Chou and M. G. Baek, Angew. Chem., Int. Ed., 2001, 40, 339 CrossRef CAS.
- A. Basso, L. Banfi, R. Riva and G. Guanti, J. Org. Chem., 2005, 70, 575 CrossRef CAS PubMed.
- D. J. Ramon and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef CAS PubMed.
- A. Domling, Chem. Rev., 2006, 106, 17 CrossRef PubMed.
- L. Weber, Drug Discovery Today, 2002, 7, 143 CrossRef CAS.
- A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3169 CrossRef.
- A. Nefzi, J. M. Ostresh and R. A. Houghten, Chem. Rev., 1997, 97, 449 CrossRef CAS PubMed.
- L. A. Thompson, Curr. Opin. Chem. Biol., 2000, 4, 324 CrossRef CAS.
- C. O. Kappe, Eur. J. Med. Chem., 2000, 35, 1043 CrossRef CAS.
- S. S. Kim, B. S. Choi, J. H. Lee, K. K. Lee, T. H. Lee, Y. H. Kim and S. Hyunik, Synlett, 2009, 599 CAS.
- K. S. Atwal, B. N. S. Wanson, S. E. Unger, D. M. Floyd, S. Mereland, A. Hedberg and B. C. J. O'Reilly, Med. Chem., 1991, 34, 806 CrossRef CAS.
- P. G. Biginelli, Gazz. Chim. Ital., 1893, 23, 360 Search PubMed.
- P. G. Biginelli, Gazz. Chim. Ital., 1893, 26, 447 Search PubMed.
- K. Joseph and S. Jain, J. Mol. Catal. A: Chem., 2006, 247, 99 CrossRef PubMed.
- K. Folkers and T. B. Johnson, J. Am. Chem. Soc., 1993, 55, 2886 CrossRef.
- U. B. More, Asian J. Chem., 2012, 24, 1906 CAS.
- J. T. Starcevich, T. J. Laughlin and R. S. Mohan, Tetrahedron Lett., 2013, 54, 983 CrossRef CAS PubMed.
- E. H. Hu, D. R. Sidler and U. H. Dolling, J. Org. Chem., 1998, 63, 3454 CrossRef CAS.
- C. O. Kappe, D. Kumar and R. S. Varma, Synthesis, 1999, 10, 1799 CrossRef PubMed.
- M. N. Esfahani, S. J. Hoseini and F. Mohammadi, Chin. J. Catal., 2011, 32, 1484 CrossRef.
- K. A. Kumar, M. Kasthuraiah, C. S. Reddy and C. D. Reddy, Tetrahedron Lett., 2001, 42, 7873 CrossRef.
- M. A. Bigdeli, G. Gholami and E. Sheikhhosseini, Chem. Lett., 2011, 22, 903 CAS.
- C. Ramalingan, S. J. Park, I. S. Lee and Y. W. Kwak, Tetrahedron, 2010, 66, 2987 CrossRef CAS PubMed.
- F. Tamaddon, Z. Razmi and A. A. Jafari, Tetrahedron Lett., 2010, 51, 1187 CrossRef CAS PubMed.
- F. Bigi, S. Carloni, B. Frullanti, R. Maggi and G. Sartori, Tetrahedron Lett., 1999, 40, 3465 CrossRef CAS.
- G. P. Romanelli, A. G. Sathicq, J. C. Autino, G. Baronetti and H. J. Thomas, Synth. Commun., 2007, 37, 3907 CrossRef CAS.
- A. Kumar and R. A. Maurya, Tetrahedron Lett., 2007, 48, 4569 CrossRef CAS PubMed.
- N. Sharma, U. Kumar-Sharma, R. Kumar and A. Kumar-Sinha, RSC Adv., 2012, 2, 10648 RSC.
- J. Safari and Z. Zarnegar, RSC Adv., 2013, 3, 17962 RSC.
- C. P. Fredlake, J. M. Crosthwaite, D. G. Hert, S. N. V. K. Aki and J. F. Brennecke, J. Chem. Eng. Data, 2004, 49, 954 CrossRef CAS.
- K. Rad-Moghadam, S. C. Azimi and E. Abbaspour-Gilandeh, Tetrahedron Lett., 2013, 54, 4633 CrossRef CAS PubMed.
- P. Salehi, M. Dabiri, M. A. Zolfigol and M. A. B. Fard, Tetrahedron Lett., 2003, 44, 2889 CrossRef CAS.
- S. D. Salim and K. G. Akamanchi, Catal. Lett., 2011, 12, 1153 CrossRef CAS PubMed.
- K. K. Pasunooti, H. Chai, C. N. Jensen, B. K. Gorityala, S. Wang and X.-W. Liu, Tetrahedron Lett., 2011, 52, 80 CrossRef CAS PubMed.
- S. Asghari, M. Tajbakhsh, B. Jafarzadeh-Kenari and S. Khaksar, Chin. Chem. Lett., 2011, 22, 127 CrossRef CAS PubMed.
- M. Zeinali-Dastmalbaf, A. Davoodnia, M. M. Heravi, N. Tavakoli-Hoseini, A. Khojastehnezhad and H. A. Zamani, Bull. Korean Chem. Soc., 2011, 32, 656 CrossRef CAS.
- S. Rostamnia and A. Morsali, RSC Adv., 2014, 4, 10514 RSC.
- J. Lu and H. Ma, Synlett, 2000, 63 CAS.
- J. S. Yadav, B. V. S. Reddy, R. Srinivas, C. Venugopal and T. Ramalingam, Synthesis, 2001, 1341 CrossRef CAS PubMed.
- Y. Ma, C. Qian, L. Wang and M. Yang, J. Org. Chem., 2000, 65, 3864 CrossRef CAS.
- K. A. Kumar, M. Kasthuraiah, C. S. Reddy and C. D. Reddy, Tetrahedron Lett., 2001, 42, 7873 CrossRef.
- D. Russowsky, F. A. Lopes, V. S. S. Silva, K. F. S. Canto, M. G. Montes and M. N. Godoi, J. Braz. Chem. Soc., 2004, 165 CrossRef CAS PubMed.
- R. Ghosh, S. Maiti and A. Chakraborty, J. Mol. Catal. A: Chem., 2004, 217, 47 CrossRef CAS PubMed.
- A. D. Patil, N. V. Kumar, W. C. Kokke, M. F. Bean, A. J. Freyer, C. D. Brossi, S. Mai, A. Truneh, D. J. Faulkner, B. Carte, A. L. Breen, R. P. Hertzberg, R. K. Johnson, J. W. Westly and B. C. M. Potts, J. Org. Chem., 1995, 60, 1182 CrossRef CAS.
- N. S. Nandurkar, M. J. Bhanushali, M. D. Bhor and B. M. Bhanage, J. Mol. Catal. A: Chem., 2007, 271, 14 CrossRef CAS PubMed.
- X.-H. Chen, X.-Y. Xu, H. Liu, L.-F. Cun and L.-Z. Gong, J. Am. Chem. Soc., 2006, 128, 14802 CrossRef CAS PubMed.
- R. Javad-Kalbasi, A. R. Massah and B. Daneshvarnejad, Appl. Clay Sci., 2012, 55, 1 CrossRef PubMed.
- Y. Qiu, H. Sun, Z. Ma and W. Xia, J. Mol. Catal. A: Chem., 2014, 392, 76 CrossRef CAS PubMed.
- J. Safaei-Ghomi, R. Teymuri and A. Ziarati, Monatsh. Chem., 2013, 144, 1865 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07334g |
| This journal is © The Royal Society of Chemistry 2014 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700