Part II: nitroalkenes in the synthesis of heterocyclic compounds
Azim Ziyaei Halimehjani
*a,
Irishi N. N. Namboothiri
*b and
Seyyed Emad Hooshmand
a
aFaculty of Chemistry, Kharazmi University, 49 Mofateh St., Tehran, Iran. E-mail: ziyaei@khu.ac.ir; Fax: +98 (21) 88820992; Tel: +98 (21) 88848949
bDepartment of Chemistry, Indian Institute of Technology Bombay, Mumbai 400 076, India. E-mail: irishi@chem.iitb.ac.in
First published on 9th October 2014
Abstract
This part (part II) is devoted to 6-membered heterocycles that are synthesized from nitroalkenes as the key substrates. These compounds can be simply accessible to form nitroalkenes and C, O, N or S-nucleophiles bearing a suitable leaving group via a wide variety of reactions such as Michael addition, hetero-Diels–Alder reaction and many cascade/domino/tandem reactions. Synthesis of six-membered saturated heterocycles such as piperidines, tetrahydropyrans, piperidinones, piperazines, tetrahydro-1,2-oxazines and thiopyrans are conveniently accessible using nitroalkenes. Also synthesis of fused 6-membered heterocycles with contiguous chiral centers, synthesis of sugar based heterocycles and aromatic heterocycles such as pyridine, pyrimidine, quinoline and quinoxaline from nitroalkenes are also presented in this part of our review.
1. Introduction
In the preceding part (part I) we have described the synthesis of 3–5 membered heterocycles from conjugated nitroalkenes as the key substrates. This part (part II) is devoted to 6-membered heterocycles that are synthesized from nitroalkenes. Synthesis of N, O or S containing heterocycles from nitroalkenes is an attractive approach not only because of the easy availability of nitroalkenes but also due to the presence of such heterocycles in bioactive molecules, including natural products.As for six-membered heterocycles with a single heteroatom, while pyridine and piperidine rings are part of many alkaloids, a tetrahydropyran ring is part of sugars and flavanoids. The thiopyran ring is also present in many bioactive compounds such as thiochromenes. Synthesis of six-membered heterocycles with multiple heteroatoms such as N, N and N, O from nitroalkenes are also described here. While pyrimidines are part of nucleosides, nucleotides and many vitamins, oxazines are precursors to unusual aminoacids, and cyclic nitronates are synthetic equivalents of 1,4-aminobutanol.
Construction of fused 6-membered heterocycles with a heteroatom (normally N) installed at the ring junction, asymmetric synthesis of functionalized and fused 6-membered heterocycles with contiguous chiral centers and synthesis of sugar based heterocycles are also part of our review. Synthesis of aromatic heterocycles such as pyridine, pyrimidine, quinoline and quinoxaline from nitroalkenes are also reviewed here.
2. N-Heterocyclic compounds
2.1. Piperidine derivatives
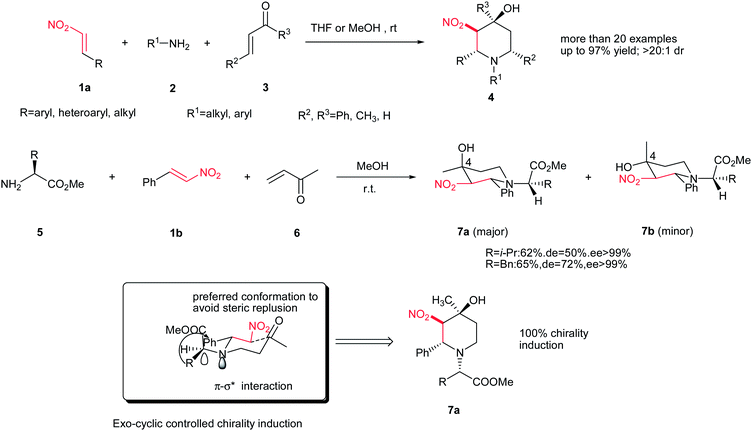
The piperidine derivatives are an attractive group of heterocycles due to their physical and biological properties and their presence in numerous naturally occurring alkaloids and synthetic compounds.1 Substituted piperidines display important biological properties such as antiviral, antidepressant, cytotoxic, antimalarial, and neuroleptic properties.2 As a result, several methods for the preparation of the piperidine ring systems are reported.3 Among them, the aza-Diels–Alder reaction4 and aza [3 + 3] cycloaddition reaction5 are straightforward methods. Recently considerable attention is denoted to the synthesis of highly substituted piperidines with focus on high efficiency and good stereoselectivity.6 In this context, the asymmetric cascade reactions are powerful tools for construction of these compounds.7Shi and coworkers demonstrated a one-pot synthesis of substituted piperidines 4 via a three component reaction occurring between a nitroalkene 1a, an amine 2, and an enone 3 in high yields and excellent diastereoselectivities without using any catalyst (Scheme 1).8 Both alkyl and aryl (heteroaryl) nitroalkenes and ketones are suitable for this cascade process. When a chiral amine 5 was used in this reaction, the corresponding enantiomeric pure piperidine 7a and 7b was obtained ingood yield and moderate diastereoselectivity (50–72% de) with only C4 isomers via an unusual exo-cyclic stereochemistry control. Proposed mechanism revealed that the process began with the aza-Michael addition of the amine to the nitroalkene to furnish a nitroalkane, which underwent a second aza-Michael addition to the enone, providing a novel nitroalkane. Finally cyclization of this nitroalkane via a nitroaldol reaction gave the corresponding substituted piperidine.
Also, polysubstituted piperidines with four contiguous stereocenters 11 were synthesized in a one step procedure with excellent enantioselectivity from protected 1-aminomethyl nitroolefins 8 and aldehydes 9 via an O-TMS protected diphenylprolinol Cat-1 catalyzed domino Michael addition/aminalization process (Scheme 2).9 Although excellent yield and stereoselectivity were obtained with linear aldehydes, with bulky isobutyraldehyde, a moderate yield and diastereoselectivity were observed without significant drop in enantioselectivity. Both alkyl- and aryl-substituted nitroolefins were compatible with this reaction. Cbz- and Boc- were used successfully as protecting group in this reaction. It is notable that the cyclic hemiaminals are valuable building blocks for elaborating other substituted piperidines 12, 13, and 14 via reduction with Et3SiH, dehydration with TFA and BF3·OEt2 mediated allylation reaction, respectively.
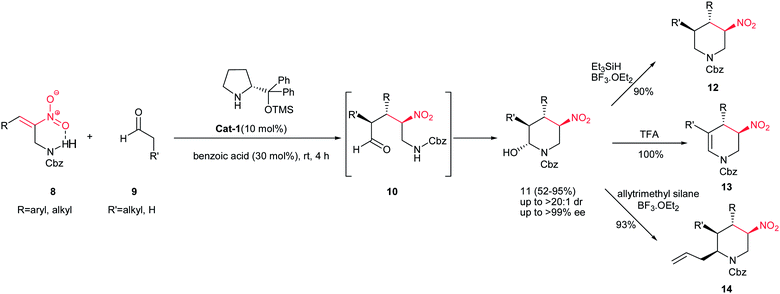 | ||
| Scheme 2 Synthesis of piperidines with four contiguous stereocenters from protected 1-aminomethyl nitroolefins. | ||
In addition, another one-pot, four-component procedure for synthesis of highly substituted piperidines 18 have been developed by Hayashi et al. via a domino Michael/aza-Henry/hemiaminalization reaction of an aldehyde 9, a nitroalkene 1, and an imine 16 followed by Lewis acid mediated allylation or cyanation (Scheme 3).10 This protocol provided diastereo- and enantioselective access to piperidines 18 with five contiguous stereocenters. Not only allylsilane and silyl cyanide but also allyl alcohol can be successfully employed as a nucleophile to afford 2-allyloxy piperidine as a β-isomer stereoselectively. The Ns (2-nitrophenylsulfonyl) protecting group on the nitrogen can be easily removed using PhSH/K2CO3 in acetonitrile to provide the deprotected piperidine rings. The relative configuration of products was determined from coupling constants and a NOESY spectrum.
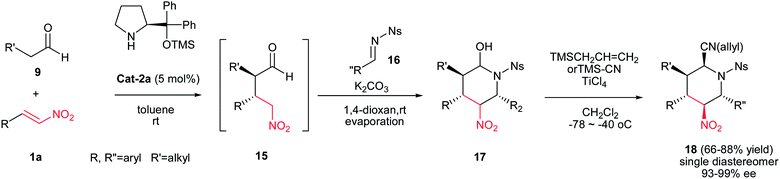 | ||
| Scheme 3 Synthesis of chiral piperidines via a one-pot Michael/aza-Henry/hemiaminalization/allylation or cyanation process. | ||
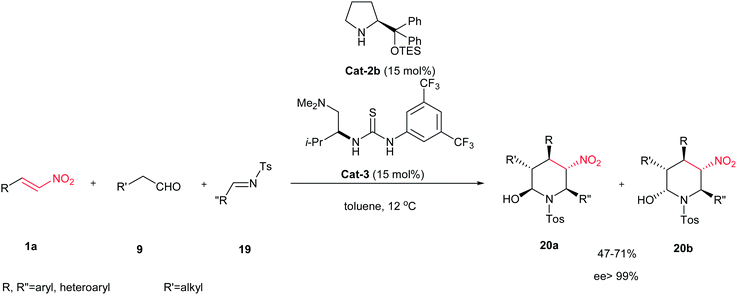
In 2010, the same strategy was applied by Xu et al. for a chemo-, diastereo- and enantioselective synthesis of fully substituted piperidines 20a/20b by using a combination of chiral diphenylprolinol triethylsilyl ether Cat-2b and a chiral thiourea Cat-3 as catalyst system.11 The process provided the piperidines in good yields, and in general almost complete enantioselectivities (>99% ee) as mixtures of α- and β-diastereomers arising from the hemiaminal stereocenter, as shown in Scheme 4. The merit of this process is highlighted by its high efficiency in producing three new bonds and five new stereogenic centres in one operation.
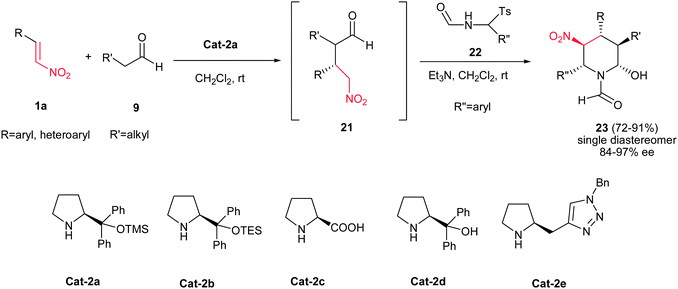
The N-formyl piperidines are valuable compounds as their CHO group can be utilized for the direct synthesis of various analogs.12 In this context, Yadav et al. demonstrated an efficient synthesis of N-formyl piperidines 23 with five contiguous chiral centers as single diastereomers via an organocatalytic Michael/aza-Henry/hemiaminalization reaction cascade of an aldehyde 9, a nitroalkene 1a, and an (aryl(tosyl)methyl) formamide 22 (simply furnished the corresponding imine via treatment with Et3N) (Scheme 5).13 Although all the pyrrolidine-based organocatalysts Cat-2a–e listed in Scheme 5 are efficient for this transformation, the best yield and stereoselectivity were obtained by diphenyl prolinol trimethyl silyl ether Cat-2a for creation of two C–C and one C–N bonds in a one-pot operation. The merits of this protocol include no by-product formation, operational simplicity, and high enantioselectivity. The relative stereochemistry of 23 was determined by NOE experiment and coupling constants.
2.2. Piperidinone derivatives
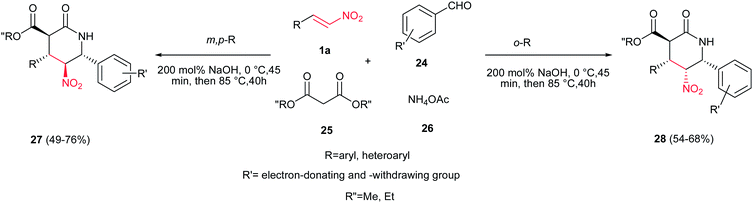
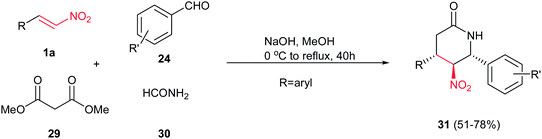
Wang et al. disclosed an interesting approach toward polysubstituted 2-piperidinones 27 and 28 via four-component reaction between substituted nitrostyrenes 1a, aromatic aldehydes 24, dialkylmalonates 25 and ammonium acetate 26 (Scheme 6).14 Reactions were carried out by stirring the mixture of a dialkylmalonate 25 (2 mmol), a nitrostyrene 1a (2 mmol), and sodium hydroxide (160 mg, 4 mmol) in 15 mL of methanol or ethanol at 0 °C for 45 min, followed by increasing the reaction temperature to rt and addition of an aromatic aldehyde 24 (2 mmol) and ammonium acetate 26 (230 mg, 3 mmol), and refluxing the reaction mixture for 40 h. The electronic properties of substituents on the nitroalkenes and aldehydes do not have significant effect on the reaction yield. It is interesting that depending on the position of substituents on the structure of aromatic aldehydes, different stereoisomers can be obtained under similar reaction conditions. While aldehydes with a substitution on the meta or para position, thiophen-3-carbaldehyde and pyridine-4-carbaldehyde afforded methyl (±)-trans-4,6-bis(aryl)-5-nitro-2-oxopiperidine-3-carboxylate 27 in this protocol exclusively, ortho-substituted aromatic aldehydes and 2-naphthaldehyde provided the (±)-trans(C3/C4)-cis(C4/C5/C6)-4,6-diaryl-5-nitro-2-oxopiperidine-3-carboxylates 28 as the only product.The same group also demonstrated that by replacing ammonium acetate with formamide 30 in this protocol, the trans-4,6-diaryl-5-nitropiperidin-2-ones 31 can be achieved in high yields. The only variation in the reaction conditions is using four equivalents of NaOH (Scheme 7).15
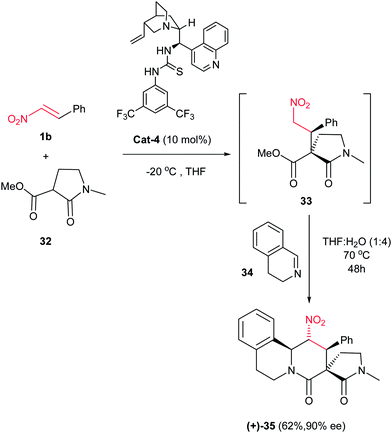
Multicyclic piperidinone ring-containing compounds 35 were synthesized by Dixon and coworkers in 2008 via a one-pot three-component Michael/nitro-Mannich/lactamization cascade. They reported that β-nitrostyrene 1a underwent Michael addition reaction with (±)-32 in the presence of 10 mol% of bifunctional catalyst Cat-4 in THF at −20 °C to furnish the intermediate 33 which underwent nitro-Mannich/lactamization reaction with imine 34 to give the desired product 35 in 62% isolated yield as a single diastereoisomer with an ee of 90% (Scheme 8).16
2.3. Pyridines & their reduced derivatives
Pyridine derivatives are an important class of aza heterocyclic compounds which are present as skeletal moieties of many natural products and biologically active compounds such as NAD nucleotides, pyridoxol (vitamin B6), and pyridine alkaloids.17 These compounds are widely used as antimalarials, vasodilators, anesthetics, anticonvulsants, antiepileptics, antioxidants, fungicidals, pesticidals, herbicidals, anthelmintics, antibacterials, antiparasitics, and dyes.181,4-Dihydropyridines as partially saturated pyridine derivatives are the most important calcium channel modulators and several derivatives of these compounds such as amlodipine, felodipine, isradipine, lacidipine, nicardipine, nifedipine, nimodipine, nitredipine etc. have been commercialized.19 Due to the wide application of pyridine derivatives, their synthesis has attracted much attention, and several protocols have been reported in literature.20 Among these, Hantzsch pyridine synthesis,21 condensation of amine and carbonyl compounds,22 multicomponent reaction of aldehydes with malononitrile23 or acetophenone derivatives24 in the presence of nitrogen source, [4 + 2] inverse electron demand aza-Diels–Alder reaction between a dienophile25 and 1,2,4-triazine, and Bohlmann−Rahtz pyridine synthesis are the most convenient approches.26
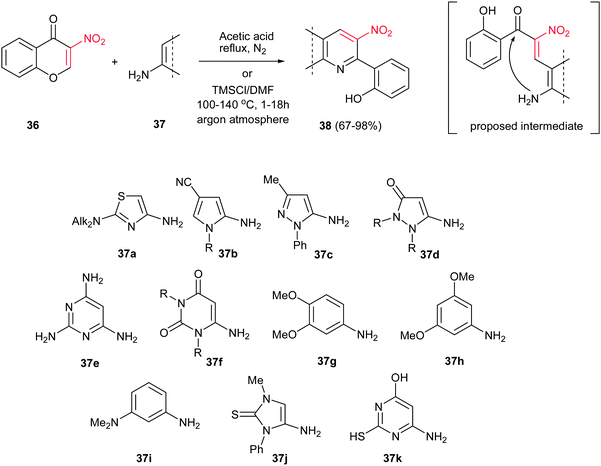
Nitroalkenes have proven to be efficient starting materials for synthesis of pyridine derivatives. In this context, Iaroshenko and Langer and co-workers reported a facile and general procedure for synthesis of various fused pyridines 38 from the reaction of 3-nitrochromone 36 with electron-rich aminoheterocycles or anilines 37 (Scheme 9).27 While for aminoheterocycles, the reaction was performed in glacial acetic acid at reflux temperature under inert atmosphere, for anilines a mixture of DMF and TMSCl was used to promote the reaction at 100–140 °C. Variety of 1,3-C,N-dinucleophiles were applied in this protocol and afforded high to excellent yields. 2-Alkyl-3-nitrochromones, 3-nitroflavones, and 3-nitrothiochromone were also evaluated without any results. It is notable that the products precipitated in the reaction mixture and collected by simple filtration. Finally, the nitro group in the products was reduced to give the corresponding 3-aminopyridine derivatives.
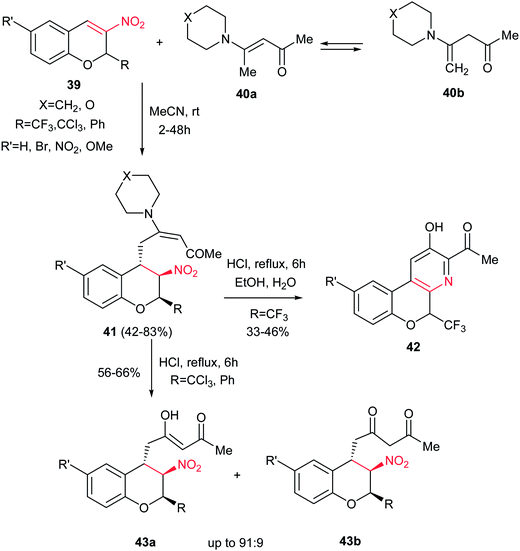
Very recently, the same group reported another interesting approach for synthesis of 5-(trifluoromethyl)-5H-chromeno[3,4-b]pyridines 42 from Michael adducts 41 of 3-nitro-2H-chromenes 39 and aminoenones 40 (Scheme 10).28 The enaminones were prepared from acetylacetone and cyclic amines. The reaction is very sensitive to the substitutions on the 2-position of chromanes. While the 2-CF3-39 afforded the corresponding fused pyridines 42 in good yields after acid hydrolysis in aqueous ethanol at reflux temperature in the presence of concentrated HCl, the 2-Ph(CCl3)-39 gave the 4-acetoacetonyl-3-nitro-2-(trichloromethyl/phenyl)chromanes 43a/43b under similar conditions. Notably, the cyclization step tolerated both electron-withdrawing and electron-donating groups on the benzene ring.
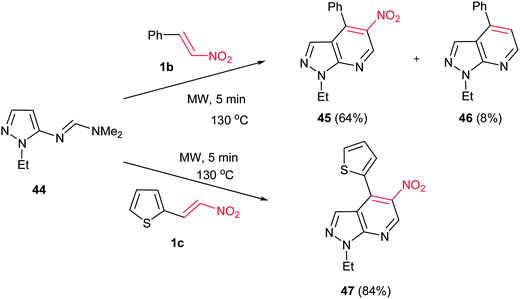
DelaHoz and co-workers reported the [4 + 2] cycloaddition reaction of (E)-N′-(1-ethyl-1H-pyrazol-5-yl)-N,N-dimethylformamidine 44 with nitroalkenes 1b/1c and it represents a new, interesting, and versatile approach for construction of pyrazolo[3,4-b] pyridines 45–47 promoted by microwave irradiation under solvent-free conditions within 5–10 min (Scheme 11).29 Under classical heating conditions, only trace amount of adduct was detected in long reaction time.
A one-pot procedure for synthesis of spirooxindole-containing fused 1,4-dihydropyridine derivatives 52 were reported by Alizadeh et al. via a pseudo five-component condensation reaction of isatin or its derivatives 50, 1,1-bis(methylthio)-2-nitroethylene 49, 1,3-dicarbonyl compounds 51, and two equivalents of ammonia 48 in the presence of 10 mol% p-toluenesulfonic acid (PTSA) in water (Scheme 12).30 1,3-Cyclohexanedione, 5,5-dimethyl 1,3-cyclohexanedione, and 1,3-dimethylbarbituric acid were successfully used as a 1,3-dicarbonyl compound. According to the proposed mechanism by the authors, the reaction initiated with the addition of ammonia to 49 to give the corresponding enamine, followed by the Stork enamine alkylation of the prepared enamine with in situ prepared Knoevenagel product from 50 and 51. Finally, imine–enamine tautomerization and loss of water afforded the product 52. The most advantages of this protocol are the use of water as a green solvent, and inexpensive and easily available starting materials and catalyst.
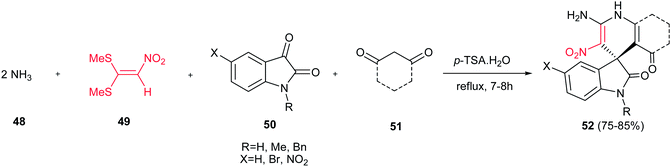 | ||
| Scheme 12 Synthesis of spirooxindole-containing fused 1,4-dihydropyridine derivatives from 1,1-bis(methylthio)-2-nitroethylene. | ||
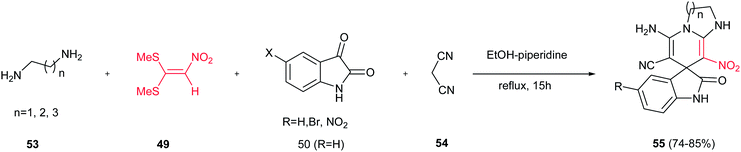
The same group developed another approach for construction of spirooxindole derivatives containing 1,4-dihydropyridine-fused 1,3-diazaheterocycle fragments 55 via a four-component reaction from 1,1-bis(methylthio)-2-nitroethylene 49, 1,n-diamine 53, isatin or its derivatives 50, and malononitrile 54 (Scheme 13).31 The reaction proceeds in ethanol in the presence of 10 mol% of piperidine under reflux conditions for 15 h. Diversity of diamines and isatins were examined to give the corresponding products in high yields. The presence of a number of functional groups in the product, potentially leads to the biological activity of the title compounds. The advantages of the present procedure are simplicity of the operation, high yields of products and easy workup and purification.
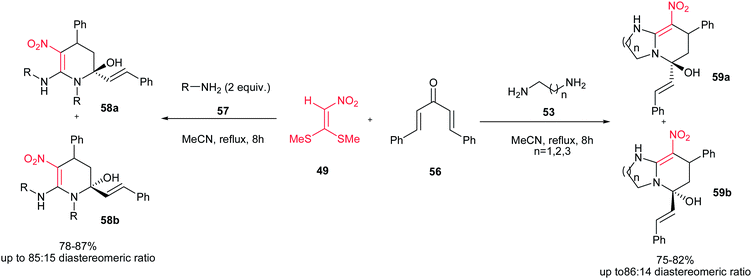
They also described another one-pot reaction for synthesis of substituted pyridinols 58a/58b from primary amines 57, nitro ketene dithioacetal [1,l-bis(methylthio)-2-nitroethene] 49 and dibenzylideneacetone 56. With using an 1,n-diamine 53 instead of primary amine, the corresponding pyrido[1,2-a]-fused 1,3-diazaheterocycles 59a/59b were also obtained in excellent yields and good diastereoselectivities (Scheme 14).32 The reaction proceeds via a condensation of amine (2 equiv.) or diamine and l,l-bis(methylthio)-2-nitroethene, followed by Michael addition reaction and hemiaminalization cascade. The most advantages of this procedure are broad application scope, excellent yields, mild reaction conditions, the ready availability of the starting materials, simple operation and easy workup.
Very recently, Prajapati et al. described that treatment of N,N-dimethyl-N′-(1-methyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl)-formimidamide 60 with an equimolar amount of nitrostyrene 1a in water at 60 °C and TBAB (10 mol%) gave, after elimination of dimethylamine from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cycloadduct and tautomerism, the corresponding dihydropyrido [2,3-d] pyrimidine derivatives 61 as the only product in high yields (Scheme 15).33 Without PTC, the reaction gave lower yield in longer reaction time. This reaction did not proceed with aliphatic nitroalkenes.
1 cycloadduct and tautomerism, the corresponding dihydropyrido [2,3-d] pyrimidine derivatives 61 as the only product in high yields (Scheme 15).33 Without PTC, the reaction gave lower yield in longer reaction time. This reaction did not proceed with aliphatic nitroalkenes.
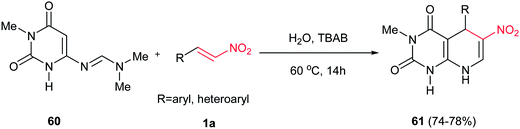 | ||
| Scheme 15 Synthesis of dihydropyrido [2,3-d] pyrimidine derivatives via Diels–Alder cycloaddition/elimination and tautomerization reaction. | ||
2.4. Pyrimidine derivatives
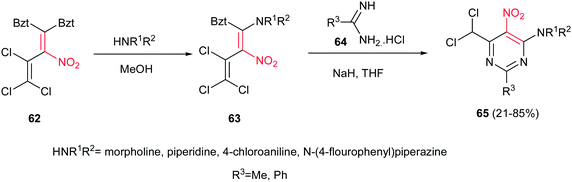
Pyrimidine and fused heterocyclic pyrimidine derivatives are interesting due to their presence in the structure of nucleosides, nucleotides, and vitamins like thiamine, riboflavin, folic acid and many other natural products.34 Compounds containing pyrimidine rings have been reported as anti-microbial, analgesic, anti-viral, anti-inflammatory, anti-HIV, anti-tubercular, anti-tumour, anti-neoplastic, anti-malarial, diuretic, cardiovascular agents.35Fully substituted pyrimidines 65 were synthesized from 1,1-bis(1H-benzotriazol-1-yl)-3,4,4-trichloro-2-nitrobuta-1,3-diene 62 in two steps. The first step is replacement of a benzotriazole group by an amine, followed by addition of an amidine 64 to afford the 2-substituted-6-(dichloromethyl)-4-amino-5-nitropyrimidines 65 in moderate to high yields (Scheme 16).36 Further transformations such as reduction of the nitro group and conversion of dichloromethyl group in the structure to cyano and triazole functionalities were also achieved.
2.5. Piperazine derivatives
β-Nitroacrylates 66 were used as suitable starting materials for synthesis of 3-substituted 5-oxopiperazine-2-carboxylate derivatives 69 by Ballini et al. in two steps.37 The first step included the Michael addition of a glycine ester 67 to a β-nitroacrylate 66 in the presence of Amberlyst A21 in ethanol to give the corresponding adducts 68 in high yield as diastereomeric mixture. This mixture was applied in the second step without further purification and reduced by Raney-Ni and H2 (2 atm) in ethanol at room temperature to afford the corresponding diastereomeric mixture of piperazines 69 via a domino reduction/cyclization process. Using chiral amino esters such as 70 and 71 in this protocol afford the corresponding products in similar yields without improving the diastereomeric ratio (Scheme 17).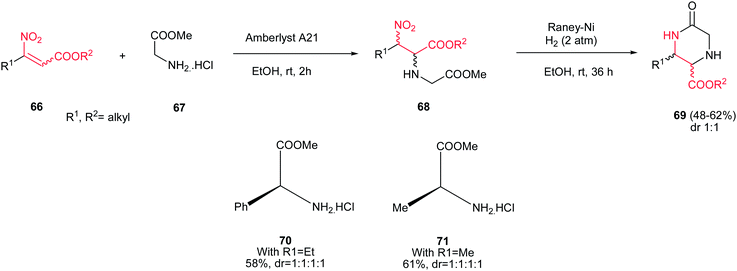 | ||
| Scheme 17 Synthesis of 3-substituted 5-oxopiperazine-2-carboxylates from aminoacids and β-nitroacrylates. | ||
2.6. Quinolines & their reduced derivatives
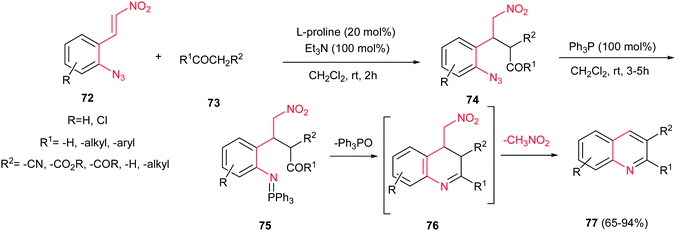
Quinolines and their reduced derivatives occur in several natural compounds and pharmacologically active substances which possess wide biological applications such as antimalarial,38a antibacterial,38b antifungal,38c anthelmintic,38d cardiotonic,38e anticonvulsant,38f anti-inflammatory,38g antioxidant,38h antidepressive38i and analgesic38j activities.38 In addition, they have been applied in photonic materials and redox switches.39 Numerous methods are available for the formation of quinoline and its derivatives such as Skraup, Dobner–von Miller, Conrad–Limpach, Friedlaender, Povarov, aza-Diels–Alder reaction, alkynylation−cyclization reactions and Pfitzinger syntheses.40Recently synthesis of these compounds by one-pot multicomponent cascade reactions have been extensively developed.41 In this context, reactions of ortho-azido-β-nitrostyrenes 72 with various carbonyl compounds 73 were investigated by Shi et al. for preparation of highly substituted quinolines 77 (Scheme 18).42 Selected conditions for this reaction included stirring 1.2 equiv. of carbonyl compounds 73 with 1 equiv. of 72 in the presence of 20 mol% of L-proline and 100 mol % Et3N in CH2Cl2 at room temperature for 2 h, followed by addition of Ph3P and additional stirring for 3–5 h at the same temperature. The reactions proceeded via a cascade Michael/Staudinger/aza-Wittig/aromatization reaction to afford the quinolines 77 in high to excellent yields (65–94%). Diversity of carbonyl compounds such as β-carbonyl carboxylates, 1,3-diketones, diethyl malonate, ethyl cyanoacetate, cyclic and acyclic ketones, and aldehydes were used successfully in this process. When isobutyraldehyde was used, the intermediate 76 was isolated as the only product. In addition, good regioselectivity was observed using 2-butanone as an unsymmetrical ketone.
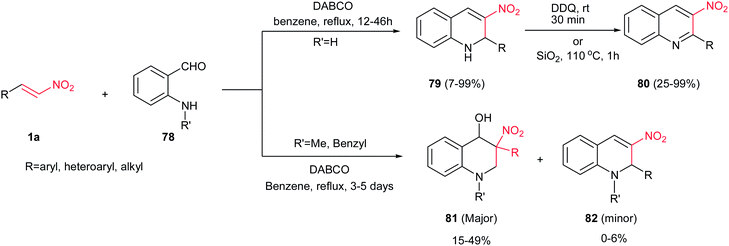
Yao et al. investigated the reaction of β-nitrostyrenes 1a with 2-aminobenzaldehyde 78 in the presence of DABCO for synthesis of 2-aryl-3-nitro-1,2-dihydroquinolines 79. The products 79 were achieved in high to excellent yields (Scheme 19).43 Aliphatic, aromatic and heteroaromatic nitroalkenes are well tolerated in this protocol. These products can be simply converted to their 3-nitro-2-substituted-quinolines 80 via in situ treatment with DDQ at room temperature or silica gel at 110 °C in high yields. With using N-alkyl 2-aminobenzaldehydes (alkyl = methyl or benzyl), the rearrangement products 81 were obtained in moderate yields as major product.
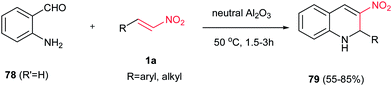
In addition, a green and efficient method for synthesis of 3-nitro-1,2-dihydroquinolines 79 is developed by Ballini et al. via condensation of 2-amino benzaldehyde 78 (R′ = H) and niroalkenes 1a in the presence of neutral alumina at 50 °C for 3 h. Both aliphatic and aromatic nitroalkenes were examined with excellent yields (Scheme 20).44
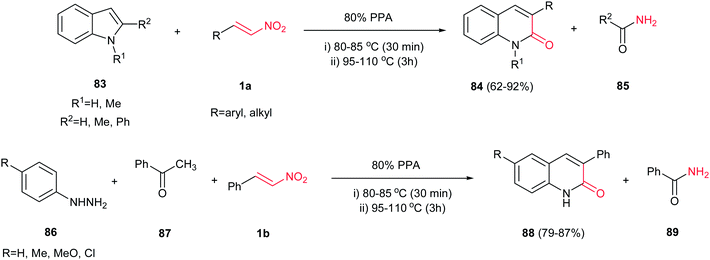
An interesting strategy for synthesis of 3-substituted 2-quinolones 84 is reported by Aksenov et al. via a transannulation reaction of 2-substituted indoles 83 with nitroalkenes 1a in polyphosphoric acid (Scheme 21).45 Reactions were performed by heating of a mixture of an indole 83 and a nitroalkene 1a in 80% PPA at 80–85 °C for 30 min and then at 95–110 °C for additional 2.5–3 h. Indole, 1-methylindole, 2-methylindole and 2-phenyl indole were used in this procedure with similar results. Aliphatic nitroalkenes gave lower yield than aromatic nitrolakenes. The only by-product of this procedure is the corresponding amide 85 of substitution on the 2-position of indole (for example acetamide from 2-methyl indole; formamide from indole, etc.), which can be simply removed by aqueous work-up. In addition, a one-pot three-component synthesis of these products was also developed by the same group via combination of this methodology with Fischer indole synthesis. They have shown that heating of a hydrazine 86, acetophenone 87 and nitrostyrene 1b in 80% PPA afforded the corresponding 3-aryl-2-quinolones 88 in excellent yields.
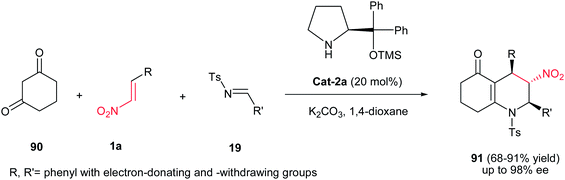
Polyfunctionalised octahydroquinolines 91 having three contiguous chiral centers were synthesized by Yadav et al. in moderate to excellent yield (68–91%) (Scheme 22).46 This one-pot reaction initiated with Michael addition of 1,3-cyclohexanedione 90 to nitroalkene 1a catalyzed by diphenyl prolinol silyl ether Cat-2a, followed by potassium carbonate-promoted aza-Henry reaction with N-tosylaldimines 19, intramolecular hemiaminalisation and dehydration reaction cascade. Nitroalkenes and imines with a phenyl group having either an electron-donating or electron-withdrawing substituent can be successfully employed in this reaction. Operational simplicity, ambient temperature and high stereoselectivity are the most advantages of this procedure. The relative stereochemistry of products was established by NOE experiments and coupling constants. The strong NOE between 4-H and 2-H suggests that they are on the same face of the molecule, that is, cis to each other. Furthermore, the absence of any measurable NOE between 2-H and 3-H indicates that 2-H and 3-H are in trans relationship.
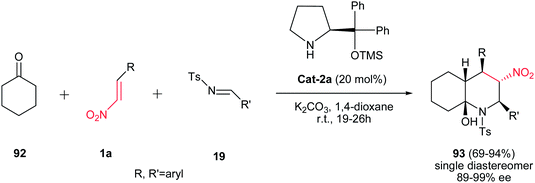
Also, the same group described that by using cyclohexanone 92 instead of 1,3-cyclohexanedione in the same reaction conditions, cis-decahydroquinolines 93 can be obtained in excellent yield and high enantio- and diastereoselectivity (Scheme 23).47 The highlight of this approach is stereoselective installation of five contiguous chiral centers into the quinoline structure. Other advantages of this procedure are operational simplicity, no by-product formation and performing reaction in ambient temperature.
2.7. Tetrahydroisoquinoline derivatives
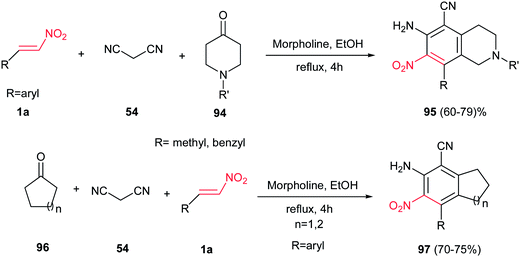
In 2011, Menendez et al. described the reaction of N-methyl(benzyl)piperidin-4-one 94 with β-nitrostyrenes 1a and malononitrile 54 in the presence of morpholine for preparation of the 6-amino-8-aryl-2-methyl(benzyl)-7-nitro-1,2,3,4-tetrahydroisoquinoline-5-carbonitriles 95 in high yields (Scheme 24).48 After screening several organic and inorganic bases and protic and aprotic solvents, using morpholine and ethanol was selected as optimum conditions in this protocol. Organic bases gave higher yield than inorganic bases under similar conditions. No reactions were performed in polar aprotic solvents such as DMSO and DMF. Replacing the 94 with cyclohexanone or cyclopentanone 96, under similar reaction conditions, afforded the corresponding indanes (n = 1) and tetralines (n = 2) 97 in high yields. According to the proposed mechanism by the authors, the reaction initiated by the Knoevenagel condensation of the starting 1-alkylpiperidin-4-one 94 with malononitrile 54, yielding an α,β-unsaturated nitrile, which then undergoes a sequential Michael addition-intramolecular Thorpe–Ziegler cyclization–tautomerization reactions with 1a. Finally, aromatization accomplished by dehydrogenation to afford compounds 95.2.8. Quinoxalines & their derivatives
Quinoxalines and their derivatives represent an important class of nitrogen-containing heterocycles which display diverse biological and pharmacological activities such as insecticide, fungicide,49 herbicide,50 anti-malarial,51 antibacterial,52 antiprotozoal,53 anticancer,54 antidepressant,55 antibiotic,56 anti-thrombotic,57 analgesic and anti-inflammatory58 properties. Reduced quinoxalines are also compounds of great biological interest.59 For example, related 3,4-dihydroquinoxalines were used as inhibitors of cholesteryl ester transfer proteins.60Despite the importance of these heterocycles in biologically important compounds, their synthetic routes are limited. These compounds usually are accessible via hydrogenation of the corresponding quinoxalines by transition-metal catalysis.61 Other main strategies are based on cyclization of o-phenylenediamines or its nitro equivalents with different substrates such as acetylenedicraboxylates,62 α-functionalized ketones and esters,63 anisylidine pyruvic acid,64 dicarbonyl compounds65 and 2,3-epoxyaldehydes.66
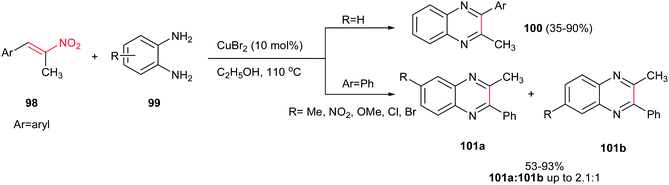
Chen and co-workers described an efficient procedure for synthesis of quinoxalines 100 via CuBr2 (10 mol%)-catalyzed reaction of o-phenylenediamines 99 and α,β-substituted nitroalkenes 98 in ethanol at 110 °C (Scheme 25).67 Nitroalkenes with different electron-donating and – withdrawing groups on the phenyl ring are well tolerated in this protocol. Electron-rich nitroalkenes afforded higher yield than electron-deficient ones. Heteroaromatic nitroalkenes gave no yield. Nitrostyrene gave no yield under similar conditions, but with changing the solvent from ethanol to DMSO, the corresponding product was obtained in 40% yield. Substituted o-phenylenediamines afforded the regioisomeric products 101a/101b in high to excellent yields. Performing the reaction under N2 atmosphere gave similar results, which revealed that the NO2 group played the oxidant role in the last step of reaction.
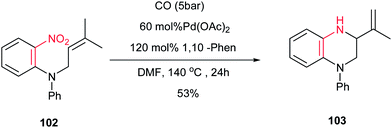
A simple procedure for synthesis of 1,2,3,4-tetrahydroquinoxaline 103 is reported by Beifuss and Merisor via reductive cyclization of ω-nitroalkene 102 in a single step by using 60 mol% of Pd(OAc)2, 120 mol% of 1,10-phenanthroline and CO (5 bar) at 140 °C in 53% yield (Scheme 26).68 They also described that this reductive cyclization system can be simply used for the synthesis of 3-isopropyl-3,4-dihydro-2H-1,4-benzoxazine from 3,3-dimethylallyl-2-nitrophenyl ether in 50% yield.
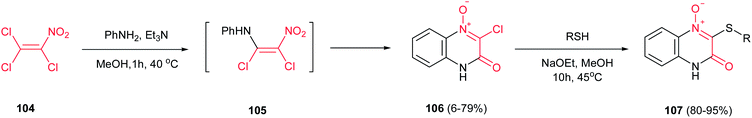
In continuation, a one-pot annulation reaction of aniline derivatives with 1,1,2-trichloro-2-nitroethene (TCNiE) 104 was reported by Kaufmann et al. to give 3-chloroquinoxalin-2(1H)-one 4-oxides 106, exclusively, in good yields (Scheme 27).69 The mode of addition plays crucial role in this reaction, in which the aniline solution (1 equiv.) must be added slowly to the solution of TCNiE (1 equiv.) in anhydrous methanol. The reaction gave lower yield for anilines substituted with electron-withdrawing groups, especially for ortho substituted ones. The chloride group in 106 can be simply substituted with thiols to improve the solubility of products and provide the novel compounds 107 with interesting properties. Further reactions of 106 with nucleophiles and electrophiles were also investigated by the same group.70
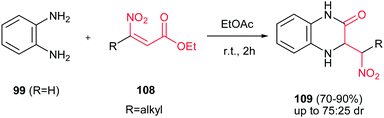
Dihydroquinoxalinones 109 were synthesized via a consecutive anti-Michael addition-amidation reaction of o-phenylenediamine 99 (R = H) with β-nitroacrylates 108 under uncatalysed reaction conditions (Scheme 28).71 According to the authors finding, the best yield was obtained when 1.25 equivalents of diamine reacted with 1 equivalent of nitroalkene in EtOAc for two hours at room temperature.
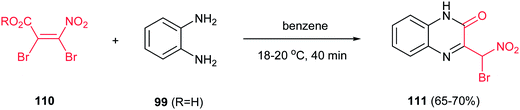
Finally, quinoxalinones 111 can be prepared via the condensation reaction of alkyl 2,3-dibromo-3-nitroacrylates 110 and o-phenylenediamine 99 (R = H) in dry benzene (Scheme 29).72
3. O-Heterocyclic compounds
3.1. Tetrahydropyrans & dihydropyrans
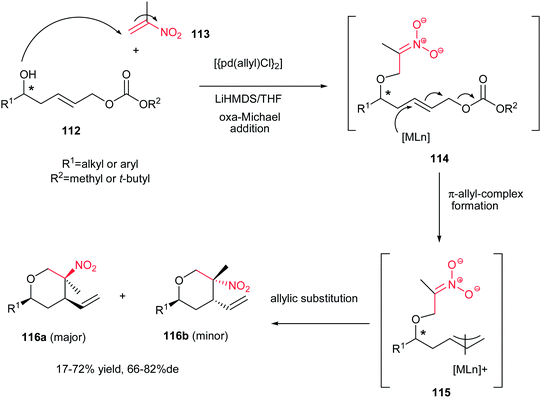
Tetrahydropyrans are common structural motifs that have been extensively used in the structure of many natural products and for the synthesis of many biologically active compounds, such as polyether antibiotics, marine toxins, pheromones, terpenoids, immunosuppressant, antitumor, antiulcer, antiproliferative, antiparasitic and anti-inflammatoryagents.73 As result, various methods were developed for synthesis of highly substituted tetrahydropyran derivatives,74 including cyclizations involving oxocarbenium ions75 and epoxides,76 hetero-Diels–Alder reaction of aldehydes with dienes or α,β-unsaturated carbonyl compounds with electron-rich carbon–carbon unsaturated bonds,77 Prins cyclizations,78 intramolecular nucleophilic reactions,79 Michael reactions,80 and one-pot procedures based on alkene–alkyne couplings followed by ether formation.81In this context, Menche and co-workers described a diastereoselective synthesis of functionalized tetrahydropyrans 116a/116b via a palladium-catalyzed domino oxa-Michael/Tsuji–Trost reaction.82 This process began with an oxa-Michael addition of chiral homoallylic alcohol 112 to a α-substituted nitroalkene 113, which generated the corresponding intermediate enolate 114. Then, this enolate furnished a π-allyl complex 115 using a catalytic amount of [{Pd-(allyl)Cl}2] combined with a base such as LiHMDS. This π-allyl complex 115 was subsequently trapped in an intramolecular fashion through an allylic substitution reaction to afford the corresponding polysubstituted tetrahydropyran 116a/116b as a mixture of two diastereomers. Among the several carbonate groups evaluated, methyl and tert-butyl carbonates proved to be the best, providing low to good yields of up to 72% combined with moderate to good relative diastereoselectivities of up to 82% de (Scheme 30). It is notable that the presence of nitro and terminal akene groups in the structure of products allows further elaboration for synthesis of complex compounds.
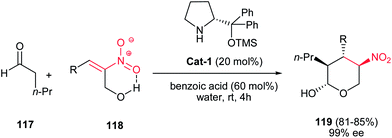
In 2011, Ma et al. developed an efficient approach for synthesis of tetrahydropyrans 119 with four contiguous stereocenters via a domino Michael addition/acetalization reaction of n-pentanal 117 and hydroxymethyl substituted nitroolefins 118 under the catalysis of Cat-1 (20 mol%) and benzoic acid (60 mol%) in water with high yields and excellent enantioselectivity (Scheme 31).9
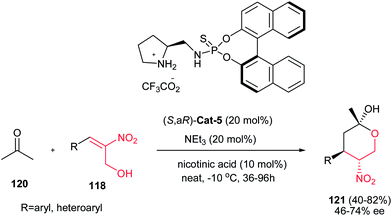
This approach was also applied by Zhou et al. for synthesis of highly substituted tetrahydropyrans 121 with three stereogenic centers. They showed that a tandem Michael addition/hemiacetalization reaction between hydroxymethyl nitrostyrenes 118 and acetone 120 catalyzed by a thiophosphoramide Cat-5 afforded the corresponding THPs 121 in high diastereo- and enantioselectivities (Scheme 32).83 The presence of an acidic cocatalyst (nicotinic acid) plays as important role in this reaction. No reaction occurred without the presence of acidic cocatalysts. The absolute configuration of the three newly generated stereogenic centers was confirmed for the product with R = phenyl by X-ray crystallographic analysis as (2R,4R,5R). This means that in chair form the relatively larger phenyl, methyl, and nitro groups occupy equatorial positions.
 | ||
| Scheme 32 Asymmetric synthesis of tetrahydropyrans via Michael addition/hemiacetalization reaction of acetone with functionalized nitroolefins. | ||
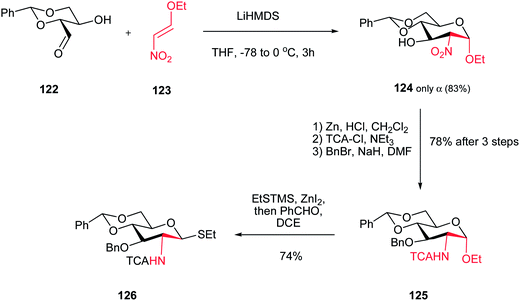
Seeberger and coworkers reported a diastereoselective synthesis of the “gluco”-configured ethyl 2-nitropyranoside 124 via domino oxa-Michael/Henry reaction between a β-hydroxy aldehyde (erythrose) 122 and 2-ethoxy nitroalkene 123 (Scheme 33).84 They have shown that the best yield and diastereoselectivity were obtained when LiHMDS was used in THF (83% isolated yield, only α isomer). Other bases such as KHMDS or NaHMDS provide lower yield and diastereoselectivity. Also, they have shown that the 2-nitro group in the product 124 can be simply reduced to the corresponding amine with zinc dust/HCl and the amine was chemoselectively protected as a trichloroacetamide in 81% yield. The free hydroxyl was also protected as benzyl ether to provide the fully protected D-glucosamine 125 which was converted to ethyl thioglycoside 126 in 74% yield using thioethyl-trimethylsilane and ZnI2 in dichloroethane.
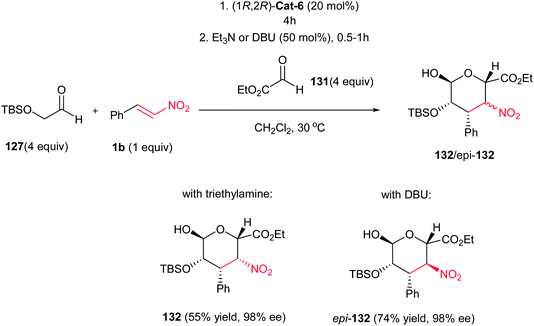
In 2010, Barbas et al. developed an efficient protocol for synthesis of carbohydrate derivatives 129 via sequential Michael–Henry reactions between aldehyde 127 and a β-nitroalkene 1a employing thiourea (1R,2R)-Cat-6 and triethylamine (Scheme 34).85 Under this catalyst system, 3,4-dideoxy-d-talose derivatives 129 were obtained in 43–76% yield with up to 98% ee, which is in equilibrium with its open form 130 in solution due to a 1,3-diaxial interaction between the nitro group and the alkoxy substituent. Indeed, only small amounts of the D-manno-isomer epi-129 were formed. Nitrostyrenes with both electron-withdrawing and electron-donating groups on the aromatic ring, heteroaromatic nitroalkenes and nitroalkenes prepared from aliphatic aldehydes were successfully used to form the desired products 129a–h (except 129e which gave the open form in 37% yield, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 dr and 99% ee) in good yields and high enantioselectivities (43–76% yield, up to >10
0 dr and 99% ee) in good yields and high enantioselectivities (43–76% yield, up to >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and up to 98% ee). When DBU was used instead of Et3N under otherwise identical conditions, complete epimerization at the stereogenic center bearing the nitro group occurred to afford the corresponding 3,4-dideoxy-D-mannose derivatives epi-129 in cyclized form (Scheme 35). By performing the reaction in the presence of glyoxylate 131, carbohydrate derivatives 132 and epi-132 were obtained upon treatment with Et3N and DBU in 55% and 74% yield respectively, but in high enantioselectivity (98% ee). (Scheme 36).85,86
1 dr and up to 98% ee). When DBU was used instead of Et3N under otherwise identical conditions, complete epimerization at the stereogenic center bearing the nitro group occurred to afford the corresponding 3,4-dideoxy-D-mannose derivatives epi-129 in cyclized form (Scheme 35). By performing the reaction in the presence of glyoxylate 131, carbohydrate derivatives 132 and epi-132 were obtained upon treatment with Et3N and DBU in 55% and 74% yield respectively, but in high enantioselectivity (98% ee). (Scheme 36).85,86
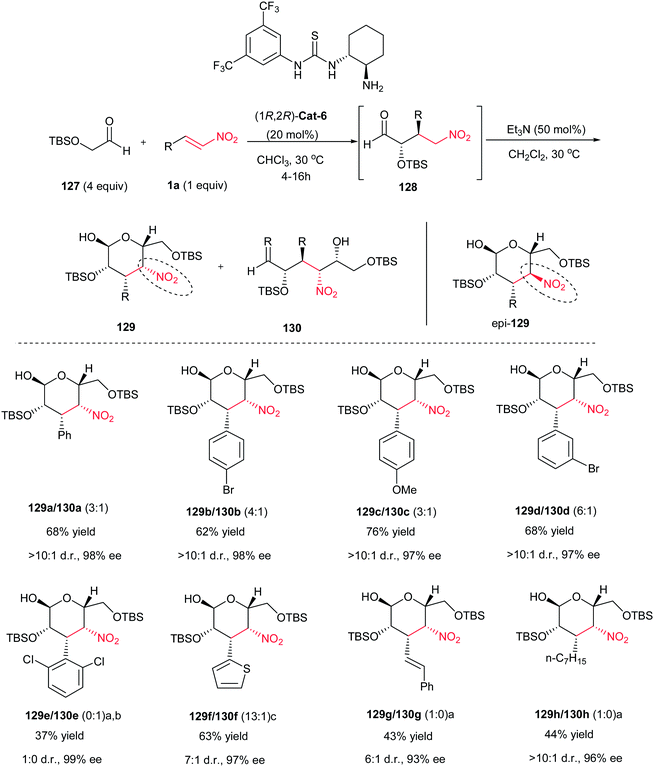 | ||
| Scheme 34 Diversity in the synthesis of 3,4-dideoxy-D-talose derivatives 129. a Reaction performed with 50 mol% (1R,2R)-Cat-6. b 100 mol% triethylamine used. c 30 mol% triethylamine used. | ||
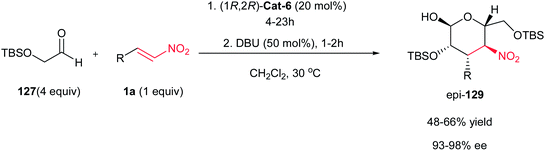 | ||
| Scheme 35 Sequential Michael–Henry reaction for the synthesis of 3,4-dideoxy-D-mannose derivatives epi-129. | ||
 | ||
| Scheme 36 Glyoxylate as aldehyde precursor in sequential Michael–Henry reaction for the synthesis of carbohydrate derivatives 132 and epi-132. | ||
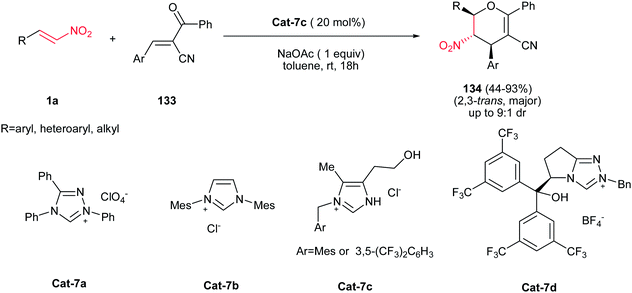
Very recently, an efficient process for synthesis of dihydropyrans containing three contiguous stereogenic centers is reported by Ye et al. via a NHC-promoted [4 + 2] cycloaddition of nitroalkenes with oxodienes (Scheme 37).87 This protocol allows access to a diversity of dihydropyrans in high yield with good diastereoselectivity. It is worth noting that the aromatic, heteroaromatic and aliphatic nitroalkenes worked well in this transformation. Among the NHC-catalysts Cat-7 was examined for this reaction, only Cat-7c afforded the best results considering both the yield and diastereoselectivity. The enantioselective version of this transformation was also performed using chiral-NHC Cat-7d in the reaction of nitrostyrene and 133, resulting the corresponding product in 24% yield and 29% ee. Based on the deuteration experiments, the authors proposed that the reaction is initiated by the addition of N-heterocyclic carbene to nitroalkene, followed by Michael addition and ring-closing O-alkylation.
3.2. Chromans and chromenes
Chromenes and chromans are important class of heterocyclic compounds due to their biological activity and their presence in a variety of significant natural products.88 An important example of a naturally occurring chromane is vitamin E, which acts as an antioxidant.89 Recently, antimicrobial, antiviral, mutagenicity, antiproliferative, sex hormone, novobiocin, antitumour, anti-cancer,and central nervous system activities of these compounds have been reported.90 Moreover, they can be used as cognitive enhancers, for the treatment of neurodegenerative diseases, including Alzheimer's disease, amyotrophic lateral sclerosis, Parkinson's disease, Huntington's disease, AIDS associated dementia and Down's syndrome as well as for the treatment of schizophrenia and myoclonus.91Consequently, due to their presence in a myriad of biologically active molecules, numerous synthetic routes towards highly functionalized chromenes and chromanes have been reported over the past decades.92 Among the reported methodologies for the synthesis of chromane derivatives, the reaction of salicylaldehyde and enolates or their equivalents has gained a prominent position.93 Other approaches include Lewis acid and transition-metal catalyzed asymmetric epoxidation,94 allylic alkylation,95 oxidative cyclization,96 enyne cyclization,97 Oxa-Povarov,98 [3 + 3] cyclo coupling of phenols and allylic alcohols,99 intramolecular arylation reactions of alkenes.100
Recently, great efforts have been devoted to the synthesis of chiral chromans, and numerous effective catalytic asymmetric approaches have been established.101 Among these methods, organocatalytic cascade reactions such as Michael–Michael, Michael–aldol and Michael–Henry reactions were considered as a straightforward and powerful processes to construct the chiral chroman framework.
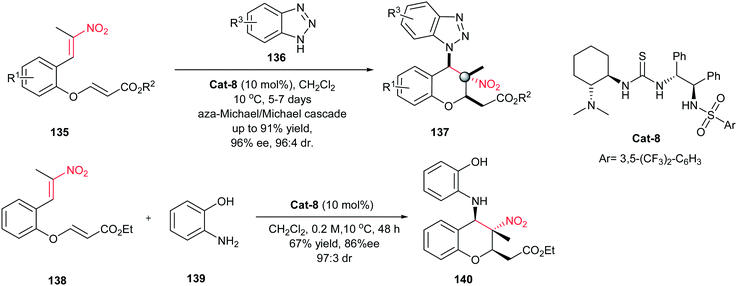
Accordingly, highly functionalized chiral chromans 137 were synthesized by Xiao et al. via a highly enantioselective cascade reaction of nitroolefin-containing enoates 135 with benzotriazoles 136 catalyzed by base/acid bifunctional organocatalyst Cat-8 (Scheme 38).102 This reaction affords chiral chromans 137 with a quaternary stereogenic center in high yield (up to 91%) with excellent enantioselectivity (up to 96% ee) and diastereoselectivity (up to 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dr). The absolute configuration of the products were determined as (2R,3S,4R) by X-ray crystallographic analysis. 2-Aminophenol 139 was also examined as a nitrogen nucleophile in this transformation to give the chroman 140 in 67% yields, 97
4 dr). The absolute configuration of the products were determined as (2R,3S,4R) by X-ray crystallographic analysis. 2-Aminophenol 139 was also examined as a nitrogen nucleophile in this transformation to give the chroman 140 in 67% yields, 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dr and 86% ee.
3 dr and 86% ee.
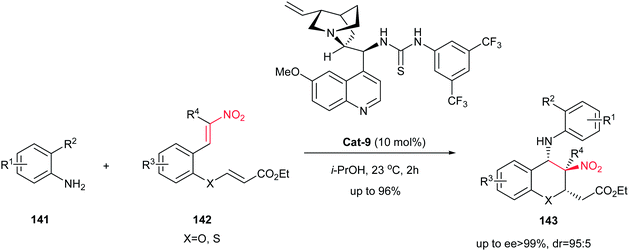
Also, the same group described that chiral bifunctional thiourea catalyst Cat-9 is suitable catalyst for asymmetric reaction of anilines 141 with nitroolefin enoates 142 for synthesis of polysubstituted chiral 4-aminochromenes 143 bearing three consecutive stereocenters in high yields with excellent stereoselectivities (Scheme 39). Also, this strategy was applied for synthesis of thiochroman derivatives with same yields and stereoselectivities.103
 | ||
| Scheme 39 Asymmetric synthesis of functionalized chromans in the presence of chiral bifunctional thiourea catalyst. | ||
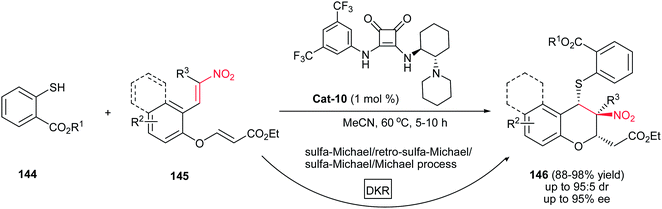
Very recently, the same strategy was applied by Du and coworkers for synthesis of chiral chromans146 via an asymmetric cascade sulfa-Michael/Michael addition reaction.104 The reaction performed well with 1 mol% of squaramide Cat-10 as catalyst in acetonitrile at 60 °C to furnish the corresponding chromans 146 in high yields with high diastereoselectivity and enantioselectivity (up to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, 95% ee) (Scheme 40). This approach furnished functionalized chromans with three contiguous stereocenters, including one quaternary center. The results show that R1 substitution has no effect on the stereoselectivity. Also linear aliphatic nitroalkene enoate such as ((2E,7E)-ethyl 8-nitronona-2,7-dienoate) was examined without any desired product. According to the observation, the authors proposed a sulfa-Michael/retro-sulfa-Michael/sulfa-Michael/Michael process, involving dynamic kinetic resolution, for this asymmetric cascade reaction.
5 dr, 95% ee) (Scheme 40). This approach furnished functionalized chromans with three contiguous stereocenters, including one quaternary center. The results show that R1 substitution has no effect on the stereoselectivity. Also linear aliphatic nitroalkene enoate such as ((2E,7E)-ethyl 8-nitronona-2,7-dienoate) was examined without any desired product. According to the observation, the authors proposed a sulfa-Michael/retro-sulfa-Michael/sulfa-Michael/Michael process, involving dynamic kinetic resolution, for this asymmetric cascade reaction.
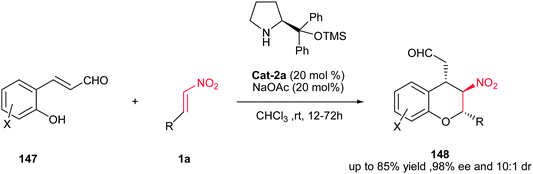
In 2009, Wang and coworkers reported an unprecedented highly enantioselective cascade oxa-Michael–Michael reaction between 2-hydroxy cinnamaldehyde 147 and trans-β-nitrostyrene 1a as substrates in the presence of 20 mol% of Cat-2a as catalyst in CHCl3 at rt (Scheme 41).105 This approach afforded a range of highly functionalized chiral chromans 148 containing multiple stereogenic centers in excellent levels of enantiomeric excess (93 to 98% ee) and good to high diastereoselectivities (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The presence of aldehyde and nitro group in product allows further elaboration for synthesis of complex molecules. Also this process was applicable to a variety of aryl- and alkyl nitroalkenes.
1). The presence of aldehyde and nitro group in product allows further elaboration for synthesis of complex molecules. Also this process was applicable to a variety of aryl- and alkyl nitroalkenes.
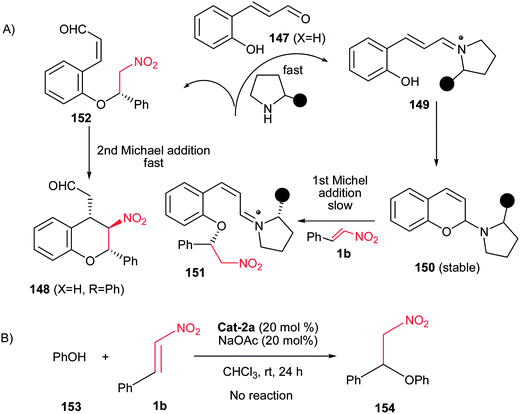
The results of preliminary mechanistic investigation by authors are given in part A in Scheme 42. They have shown that an aminal intermediate 150 is the first species in this transformation, which was formed very quickly from reaction of 147 with Cat-2a in an almost quantitative yield. This intermediate was isolated and characterized. The aminal 150 then underwent the first Michael addition to nitroolefin 1b to produce the stable intermediate 151 which was isolated and characterized completely. The Cat-2a was released for the next cycle reaction via exchange of 151 with 147, and meanwhile compound 152 was produced. Finally, an intramolecular Michael reaction in 152 gave rise to the product 148. The preparation of aminal 150 was supported by this hypothesis that under the same reaction conditions, no reaction occurred between phenol 153 and trans-β-nitrostyrene 1b (part B, Scheme 42), indicating that the “OH” of phenol was not a nucleophile for the first Michael reaction.105
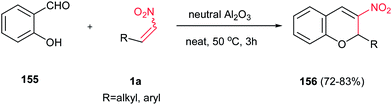
It is well known that the reaction of activated trihalomethylated alkenes with salicylaldehydes in the presence of a base gives 3-substituted 2-trihalomethylchroman-4-ols and 2-trihalomethyl-2H-chromenes106 in high yields, which turned out to be highly reactive substrates in the reactions with N-, S-,and C-nucleophiles.107 In this context, an efficient and environmentally benign procedure for synthesis of 3-nitrochromenes 156 was reported by Ballini et al. from salicyladehyde 155 and nitroalkenes 1a in the presence of neutral Al2O3 (Scheme 43).44 The best yield was obtained by using the ratio Al2O3 g mmol−1 of substrate = 1 and heating this heterogenous mixture at 50 °C for 3 h under solvent-free conditions. A variety of aryl- and alkyl nitroalkenes was examined in this reaction with high yields (72–83%). The advantages of this protocol include a simple reaction setup, short reaction times, high product yields, using cheap catalyst, elimination of solvent and no workup is needed, since the crude mixture can be directly charged to a chromatographic column for immediate purification.
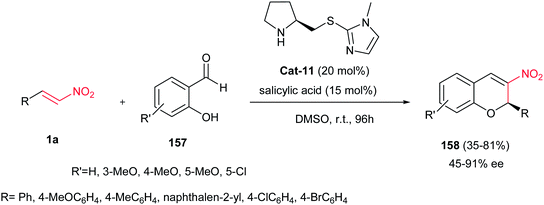
In 2008, an asymmetric version of this reaction was developed by Xu et al. using organocatalyst Cat-11 and the cocatalyst salicylic acid. The reaction proceeded between substituted salicylaldehydes 157 and nitroalkenes 1a via tandem oxa-Michael–Henry reaction to give the corresponding products 158 in moderate to good enantioselectivites (Scheme 44).108 The reaction was applicable for a wide range of salicylaldehydes and β-nitrostyrenes. The authors assumed that the organocatalyst Cat-11 may have a dual role in the process, as activator for asymmetric iminium formation and as Lewis base to facilitate the deprotonation of the salicylaldehyde. The salicylic acid may also have a dual role in the reaction, which might promote the aromatic iminium formation and form hydrogen bonding interaction between the nitro oxygen of 1a and its hydroxy group.
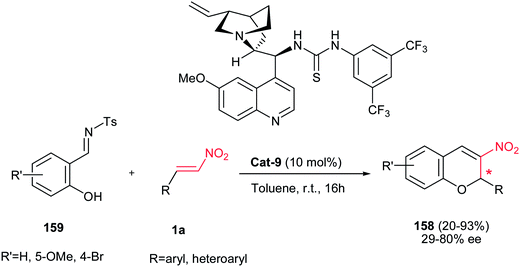
Schreiner et al. developed another asymmetric approach for the synthesis of 3-nitro-2H-chromenes 158 by reaction of salicyl N-tosylimine 159 with nitroolefins 1a promoted by bifunctional thiourea Cat-9 as the catalyst (Scheme 45).109 Aromatic nitrostyrenes substituted with electron-donating and – withdrawing groups on the phenyl ring can undergo a tandem oxa-Michael-aza-Henry-desulfonamidation process to afford the corresponding products in moderate to good yields and enantioselectivities by a kinetically controlled desulfonamidation step. By running the reaction at 0 °C higher ee was obtained with losing the yield (22–68% yield, 46–97% ee) compare to rt (34–93% yield, 29–50% ee).
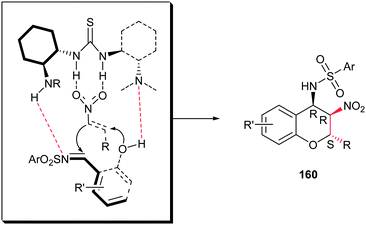
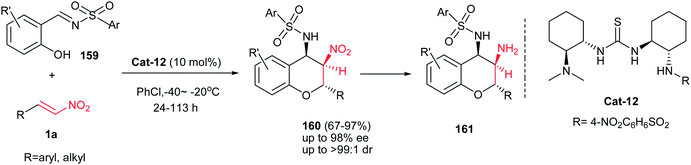
Although the Schreiner group has shown that the desulfonamidation reaction was occuredduring the reaction conditions, more recently, Peng et al. reported a simple and efficient procedure for synthesis of polysubstituted chiral 4-aminobenzopyrans 160 from the same starting materials without removing the sulfonamide group.110 The reaction proceeds via an oxa-Michael/aza-Henry cascade reaction promoted by trifunctional catalyst Cat-12 (tertiary amine, thiourea, and sulfonamide) to achieve product 160 with three consecutive stereocenters in high yield (up to 97%) with excellent stereoselectivity (up to 98% ee and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) (Scheme 46). They confirmed that the position of the substituent on the aryl ring of nitrostyrene affected the enantioselectivity and reactivity in which meta substituents gave better results in shorter reaction times than those with ortho- or para-substituents. In addition, the electronic effect of the substituent on the benzene ring of the nitroolefins 1a or salicylaldimine 159 had some influence on reactivity but only a small effect on the stereoselectivity. It is notable that the products can be simply converted to the nonsymmetric 3,4-diaminochromanes 161 via reduction of nitro group by Zn/HCl at room temperature. The authors proposed that the catalyst promotes the reaction via activation of the nitrostyrene and imine by hydrogen bonding and abstraction of the phenolic hydrogen by tertiary amine group as shown in Scheme 47.
1 dr) (Scheme 46). They confirmed that the position of the substituent on the aryl ring of nitrostyrene affected the enantioselectivity and reactivity in which meta substituents gave better results in shorter reaction times than those with ortho- or para-substituents. In addition, the electronic effect of the substituent on the benzene ring of the nitroolefins 1a or salicylaldimine 159 had some influence on reactivity but only a small effect on the stereoselectivity. It is notable that the products can be simply converted to the nonsymmetric 3,4-diaminochromanes 161 via reduction of nitro group by Zn/HCl at room temperature. The authors proposed that the catalyst promotes the reaction via activation of the nitrostyrene and imine by hydrogen bonding and abstraction of the phenolic hydrogen by tertiary amine group as shown in Scheme 47.
 | ||
| Scheme 46 Synthesis of polysubstituted chiral 4-aminobenzopyrans with three consecutive stereogenic centers. | ||
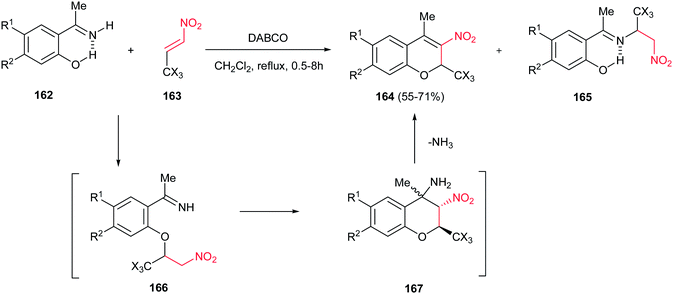
In 2008, Korotaev group proved that using 2-hydroxyacetophenone instead of salicaldehyde in the reaction with nitroalkenes failed to prepare 4-methyl-3-nitro-2-trihalomethyl-2H-chromenes 164 due to the lack of the ketone reactivity toward nucleophilic attack compared to formyl group. To overcome this problem, they reported an alternative procedure for synthesis of 4-methyl-3-nitro-2-trichloro (triflouro)-methyl-2H-chromenes 164 from reaction of N-unsubstituted imines 162 of 2-hydroxyacetophenones with trichloro(trifluoro)ethylidene nitromethanes 163 in the presence of DABCO in refluxing CH2Cl2 (Scheme 48).111 Under these conditions, the reaction proceeded via a tandem oxa-Michael/aza-Henry reaction to give the corresponding products in high yields. Although two competitive routes (oxa-Michael/aza-henry vs. Michael addition) were observed, the reaction pathways could be controlled by chosing proper solvent and reaction temperature.
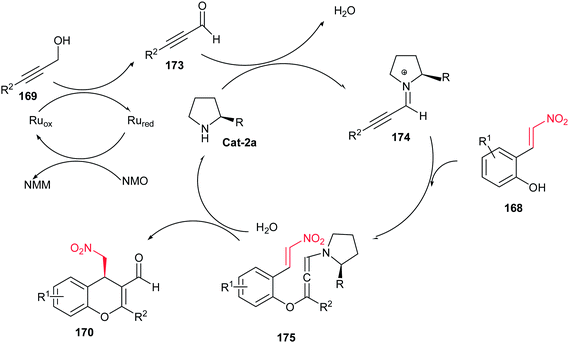
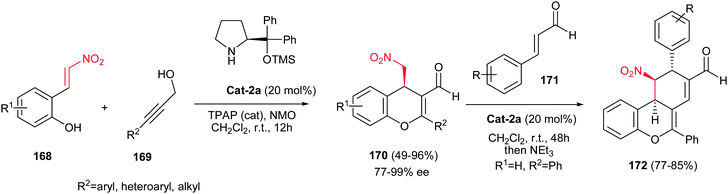
Recently, an efficient synthesis of 4H-chromene derivatives 170 has been developed from a propargyl alcohol 169 and a nitroalkene 168 derived from a cinnamaldehyde via a catalytic asymmetric oxidative iminium–allenamine cascade reaction (Scheme 49).112 Different reaction conditions were examined and 7 mol% of TPAP, NMO (1.6 equiv.), 20 mol% of Cat-2a, and 1.5 equiv. of a propargy alcohol 169 for 1 mmol of a (E)-2-(2-nitrovinyl)-phenol 168 in CH2Cl2 was selected as optimum conditions to give the corresponding products in good yield and excellent enantiomeric excess (95% ee). Also the authors proved that the TPAP/NMO redox system is not compatible with diarylprolinolsilylether catalyst Cat-2a, starting materials, different reactive functionalities and chromene products. In addition, the products were used in another domino iminium–enamine cascade reaction with cinnamaldehyde 171 to prepare the tricyclic 4H-chromenes 172 with three chiral centers in high yields.
 | ||
| Scheme 49 Asymmetric synthesis of 4H-chromenes and their application for synthesis of the tricyclic 4H-chromenes. | ||
The proposed reaction mechanism involves initially oxidation of propargyl alcohol 169 to propargyl aldehyde 173 with releasing one equivalent of N-methyl-morpholine (NMM), followed by formation of the iminium ion intermediate 174 via condensation with the secondary amine catalyst. The intermediate 174 then undergo an oxa-Michael addition with 168 to furnish the allenamine intermediate 175 which finally reacts with the nitroalkene moiety by an intramolecular Michael addition leading to compound 170 after releasing the catalyst (Scheme 50).112
In addition, a one-pot procedure for diastereo- and enantioselective synthesis of polyfunctionalized chromene derivatives starting from 2-(nitrovinyl)phenols 168 and various cyclic dicarbonyl nucleophiles was reported by Enders group. Quinine thiourea catalyst Cat-12 (10 mol%) was examined as best catalyst for this transformation (Scheme 51).113 The reaction proceeds via a domino Michael-hemiacetalization and dehydration sequence. Cyclopenta- or cyclohexa-[b]chromenes 177 were obtained when five- or six-membered β-keto ester 176 reacted with 168 in the presence of 10 mol% of Cat-9 and dehydration with 300 mol% of P2O5. While with acyclic 1,3-diketones 178, tricyclic spirochromans 179 were obtained, in the case of cyclic 1,3-diketones 180 as nucleophiles, the dehydration occurred regioselectively to provide tetrahydro-1H-xanthen-1-ones (181, n = 1) or 2,3-dihydrocyclopenta[b]chromen-1(9H)-one (181, n = 0) bearing one stereogenic center. With this procedure, chroman derivatives with several functional groups such as the nitromethyl, ester, keto, and double bonds and various substituents at the aromatic ring can be prepared.
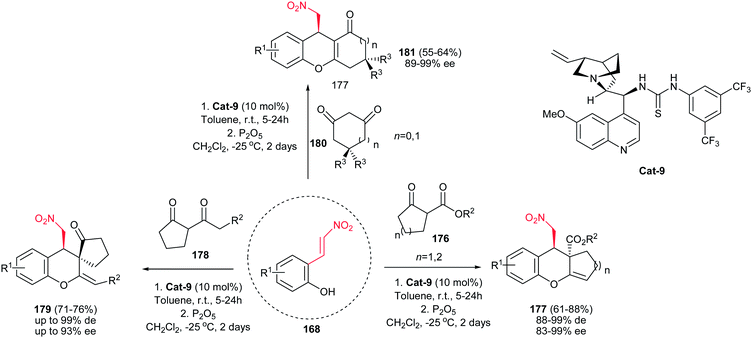 | ||
| Scheme 51 Asymmetric synthesis of chroman derivatives via one-pot domino Michael-hemiacetalization and dehydration. | ||
A similar procedure using linear β-keto esters 183 and catalyst Cat-13 was reported by the same group to provide the polyfunctionalized 4H-chromenes 185 with good to excellent yields (76–95%) and enantioselectivities ranging from 30–99% ee (Scheme 52).114
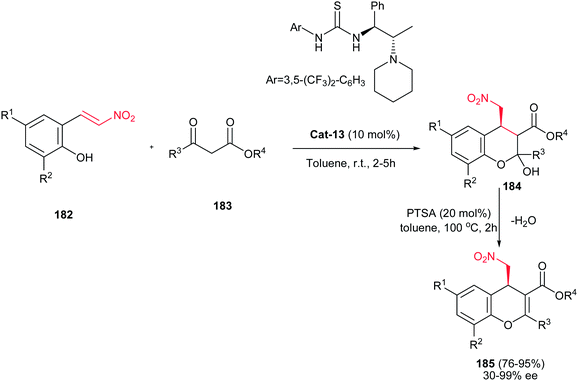 | ||
| Scheme 52 Bifunctional-thiourea organocatalyst-promoted asymmetric synthesis of polyfunctionalized 4H-chromenes. | ||
Reactions of pyridoxal hydrochloride 186 with 1-nitro-3,3,3-trichloro(trifluoro)propenes 163 in the presence of NaOH in aqueous medium were investigated by Sosnovskikh et al. in 2012.115 They described that while reaction of trifluoromethylated nitroalkenes 163 (X = F) with pyridoxal 186 at room temperature afforded the CF3-containing 7-aza-3-nitro-2H-chromenes 187 in good yields, trichloromethylated nitroalkenes need higher temperatures (45–50 °C) or additional treatment with concentrated HCl to give the corresponding CCl3-chromenes 188. Reaction of nitrostyrenes 1b with 186 under similar conditions provided a mixture of trans–cis and trans–trans azachromanols 189a/189b in 49% yield and 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 dr (Scheme 53).
41 dr (Scheme 53).
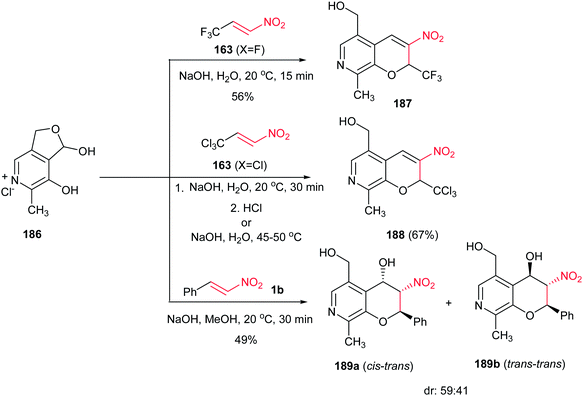 | ||
| Scheme 53 Synthesis of 7-aza-3-nitro-2H-chromenes and 7-aza-3-nitro-chromans from pyridoxal hydrochloride and nitroalkenes. | ||
Reactions of nitroketene N,S-acetals 190 and the ring substituted 2-hydroxybenzaldehydes 191 in the presence of a base were introduced by Rao and Geetha in 2009.116 Various reaction conditions using different bases, solvents and temperature were tested for this reaction and using 10 mol% of DBU in methanol at room temperature was selected as optimal conditions with regards to yields and reaction times (Scheme 54). Under the optimal conditions, diversity of salicaldehydes and nitroalkene N,S-acetals were applied to give the 2-alkylamino-3-nitro-4-alkylsulfanyl 4H-chromenes 192 in excellent yields. The reaction proceeded via a sequential oxa-Michael/intramolecular nitro-aldol condensation/dehydration and dethiomethylation/thiomethylation reactions. Notably, the alkylsulfanyl group in the structure of products can be simply displaced by various thiols via refluxing in ethanol. When NaH in THF was applied as catalyst for this reaction, the compound 193 was observed as minor product along with the major product 192.
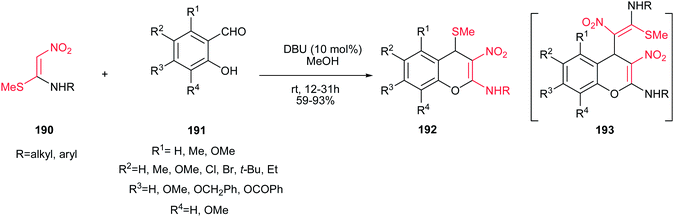 | ||
| Scheme 54 Synthesis of 2-alkylamino-3-nitro-4-alkylsulfanyl 4H-chromenes from salicaldehydes and nitroalkene N,S-acetals. | ||
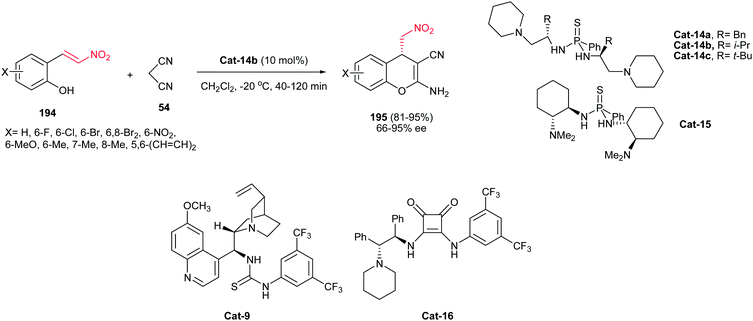
Finally, Zhou et al. developed a new series of chiral thiophosphonodiamides catalysts Cat-14 and Cat-15 for asymmetric synthesis of 2-amino-4H-chromene-3-carbonitriles 195 in high yields (up to >99%) with 66–95% ee from (E)-2-(2-nitrovinyl)phenols 194 and malononitrile 54 via a tandem Michael addition/cyclization reaction (Scheme 55).117 Reactions were performed via stirring a mixture of 2-(E)-2-nitrovinylphenols 194 (0.50 mmol) and malononitrile 54 (0.75 mmol) in the presence of 10 mol% Cat-14b in methylene chloride (2 mL) at −20 °C for 40–120 min. Performing the reaction in polar solvents such as ethanol and acetonitrile gave the racemic products. 2-(E)-2-Nitrovinylphenols bearing both electron-withdrawing and electron-donating substituents on the benzene ring are compatible in this protocol. Among the thiophosphonodiamide catalysts examined for this transformation, Cat-14b was selected as the best catalyst with regard to both the yield and the ee value. Theoretical study by authors revealed that the asymmetric induction by catalyst is the result of coordination of both nitroalkenes and the anion of malonitrile to the diamide and tertiary amino group of Cat-14b via hydrogen bonding in the transition state.
 | ||
| Scheme 55 Thiophosphonodiamides-promoted asymmetric synthesis of 2-amino-4H-chromene-3-carbonitriles. | ||
It is worth noting that this catalytic system shows higher yields and ee values compared to the previously reported results with bifunctional thiourea Cat-9 (4–91% ee)118 or squaramide catalyst Cat-16 (41–95% ee)119 at the same catalyst loading in this transformation. It is notable that using chiral bifunctional thiourea Cat-9 and squaramide Cat-16 catalysts afforded the enantiomer of 195.
4. S-Heterocyclic compounds
4.1. Thiopyran derivatives
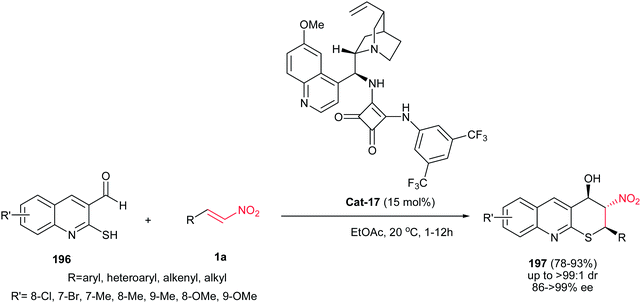
A bifunctional squaramide-catalyzed asymmetric synthesis of 2H-thiopyrano[2,3-b]quinolines with three contiguous stereocenters is reported by Zhou and co-workers (Scheme 56).120 This tandem Michael–Henry reaction was performed by stirring 2-mercaptoquinoline-3-carbaldehyde with slightly excess of a nitroalkene in EtOAc at rt or 0 °C in the presence of 15 mol% of Cat-17. The use of quinine and cinchonidine-derived thioureas afforded lower selectivities compare to Cat-17. This reaction is possible for a variety of aryl, heteroaryl, alkenyl and alkyl nitroalkenes and afford the corresponding optically active 2H-thiopyrano[2,3-b]quinolines in excellent yields, diastereoselectivities (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to >99
5 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enantioselectivities (86 → 99%). The stereochemistry of products was established as (2R,3R,4S) by X-ray analysis.
1) and enantioselectivities (86 → 99%). The stereochemistry of products was established as (2R,3R,4S) by X-ray analysis.
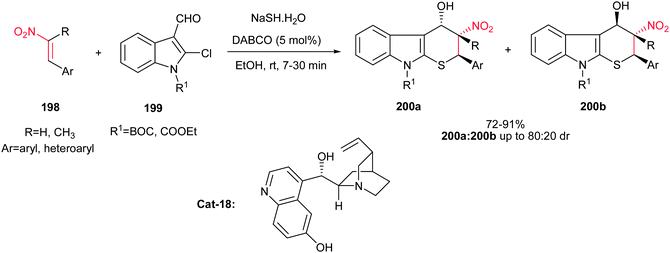
An environmentally benign procedure for the synthesis of 2-aryl-3-nitro-4-hydroxy-2,3,4,9-tetrahydrothiopyrano[2,3-b]indole derivatives 200 is reported by Samanta and co-workers.121 They demonstrated that combination of β-nitrostyrenes 198, N-protected-2-chloro-3-formylindoles 199, and sodium hydrosulfide at room temperature in ethanol using DABCO as the catalyst afforded the corresponding products 200 in high to excellent yields (Scheme 57). The reaction initiated with nucleophilic thiolation, followed by thia-Michael addition and intramolecular Henry reaction. Although three contiguous chiral centers were generated in this protocol, only two diastereomers 200a/200b were obtained as depicted in Scheme 57. By performing the reaction in nonpolar solvents such as THF, Et2O, and MeCN, reverse configuration of major diastereomer 200a can be obtained, albeit in lower yield. It is notable that in the case of 2-(2-nitrovinyl) furan, the dehydrated product was obtained in 91% yield. The asymmetric version of this reaction was performed using 5 mol% of chiral catalyst Cat-18 in a solvent mixture of CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF
THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O at −40 °C. The corresponding products were obtained in high yields (82–88%) and good diastereo- and enantioselectivities (dr up to 4
H2O at −40 °C. The corresponding products were obtained in high yields (82–88%) and good diastereo- and enantioselectivities (dr up to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for cis–trans product; ee up to 88% for major isomer).
1 for cis–trans product; ee up to 88% for major isomer).
4.2. Thiochromane derivatives
Thiochromanes, the sulfur analogues of chromanes, have attracted considerable attentions due to their presence in a variety of biologically active compounds.122 They have shown ability to act on the central nervous system receptors,123 and as antihypertensive,124 anti-HIV125 and cardiovascular agents.122a In addition, thiochromane derivatives display a mixed binding affinity to dopamine D2, D3 and 5HTA1 receptors.126Several methods have been reported for the synthesis of thiochromanes with different substitution pattern.127 Among them, classical Claisen rearrangement of allyl phenyl sulfides and acid-catalyzed intramolecular cyclocondensation of β-arylthio aldehydes and α,β-unsaturated carbonyl compounds are the most common approaches.128 Recently, several asymmetric methods have been developed for the synthesis of these compounds, which include asymmetric reduction of thiochroman-4-ones, enzymatic resolution of racemic 4-hydroxythiochromanes or 4-acyloxythiochromanes, and enantioselective alkylation of thiochromanes via conjugate addition.129
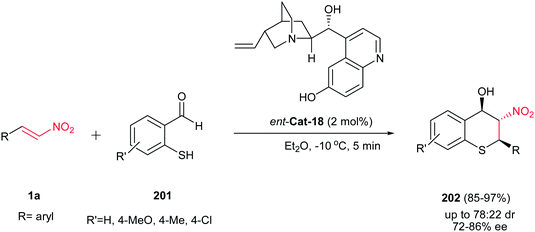
In this context, optically active thiochromanes 202 were synthesized from β-nitroalkenes 1a and 2-mercaptobenzaldehydes 201 via cascade Michael/Henry reactions by using 2 mol% of cupreine ent-Cat-18 as the catalyst (Scheme 58).130 Good enantio-and diasteremeric ratios of 2-aryl-3-nitrothiochroman-4-ols 202 were obtained in diethyl ether at −10 °C which were further improved through a single recrystallization from hexane/EtOAc (up to 98% de and >99% ee). Moreover, the nitro group was reduced to the corresponding amine using H2 and Pd/C.
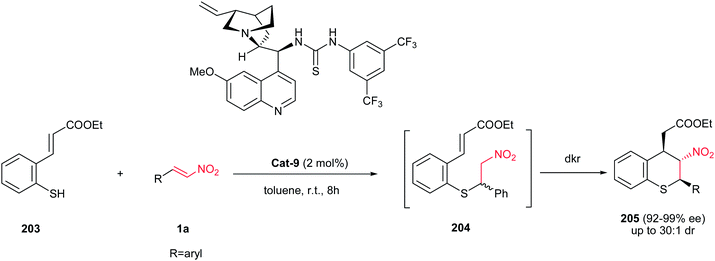
Wang and co-workers reported another highly enantio- and diastereoselective tandem Michael–Michael sequence for the synthesis of substituted thiochromanes 205. The reaction proceeds via Michael addition of thiol group of 203 to nitroalkenes 1a, followed by intramolecular Michael addition of prepared nitroalkane to the cinnamate goup in the presence of catalytic amount of Cat-9 as a chiral catalyst (Scheme 59).131 The authors observed that the Michael addition of unsubstituted thiophenols to nitroolefins proceeds with poor stereoselectivities. According to this observation, they proposed that while the thia-Michael addition is reversible and not particularly stereoselective, the high stereoselectivities observed (92–99% ee, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 30
1 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) are the result of a dynamic kinetic resolution (dkr) taking place in the second step of the reaction. To confirm this hypothesis, they have shown that in the presence of catalyst Cat-9, a racemic thia-Michael adduct was converted into the corresponding thiochromane with excellent stereoselectivity (95% ee, >30
1 dr) are the result of a dynamic kinetic resolution (dkr) taking place in the second step of the reaction. To confirm this hypothesis, they have shown that in the presence of catalyst Cat-9, a racemic thia-Michael adduct was converted into the corresponding thiochromane with excellent stereoselectivity (95% ee, >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).
5. N,O-Heterocyclic compounds
5.1. Tetrahydro-1,2-oxazines
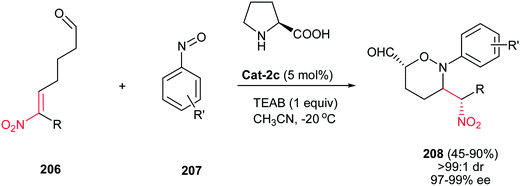
1,2-Oxazines have found considerable interest in organic synthesis as useful intermediates132 in the synthesis of natural products133 and unnatural cyclic amino acids.134 1,2-Oxazine derivatives exhibit a broad spectrum of biological activities.135 In recent years, the most applied procedures for synthesis of these compounds are included [3 + 3] cyclization,136 homo [3 + 2] dipolar cycloaddition,137 and tandem aminoxylation/aza-Michael addition reaction.138In 2008, Zhong and co-workers reported an elegant procedure for synthesis of functionalized tetrahydro-1,2-oxazines (THOs) 208 via proline-catalysed O-nitroso aldol/aza-Michael sequence (Scheme 60).139 The bifunctional nitroalkene aldehyde 206 has a dual role in this reaction as nucleophile to aryl nitroso derivatives 207 via its enamine intermediate and as electrophile through the nitroalkene moiety. The oxazine products 208 with two stereogenic centers in a 1,4-trans relationship were obtained in good yields (45–90%) and excellent stereoselectivities (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 97–99% ee).
1 dr and 97–99% ee).
 | ||
| Scheme 60 Proline-catalyzed O-nitroso aldol/aza-Michael organocascade for enantioselective synthesis of THOs. | ||
5.2. Cyclic nitronates
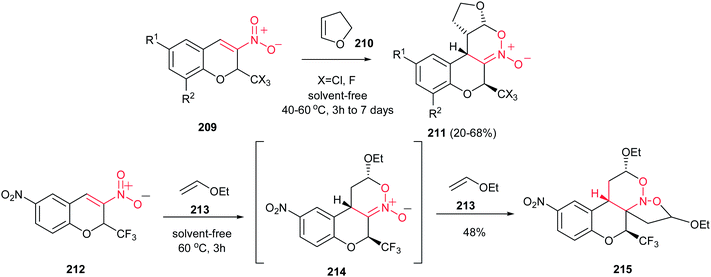
The six-membered cyclic nitronates are the most effective precursors for the synthesis of stereochemically complex acyclic and heterocyclic compounds. The value of cyclic nitronates for the directed synthesis is examined by several factors. Firstly, they can be readily synthesized in the form of individual diasteremers and enantiomers by [4 + 2] cycloaddition reaction of nitroalkenes to olefins. Secondly, the 1,2-oxazine cycle is synthetic equivalent of pyrrolidine and 1,4-aminobutanol. Finally the versatile reactivity of the nitronate fragment opens a number of possibilities for further functionalization of six-membered cyclic nitronates for synthesis of several naturally occurring compounds. The chemistry of cyclic nitronates is widely developed by Denmark group.140Hetero-Diels–Alder reaction between 3-nitro-2-trihalomethyl-2H-chromenes 209 or 212 and enol ethers 210 or 213 under solvent-free conditions is reported by Sosnovskikh et al. for synthesis of novel cyclic nitronates 211 or 214 containing CF3 and CCl3 group (Scheme 61).141 These compounds can undergo further 1,3-dipolar [3 + 2] cycloaddition reaction with another vinyl ether, leading to new fused chroman derivatives 215 of biological interest. The stereochemistry of the products was established based on 2D COSY, NOESY, HSQC, and HMBC experiments and X-ray diffraction analysis. The adduct 215 was thermally stable in CH2Cl2–hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), but it proved to be unstable in CDCl3 and DMSO-d6 solutions.
2), but it proved to be unstable in CDCl3 and DMSO-d6 solutions.
 | ||
| Scheme 61 [4 + 2] Cycloaddition route for synthesis of cyclic nitronates containing CF3 and CCl3 group and their further elaboration. | ||
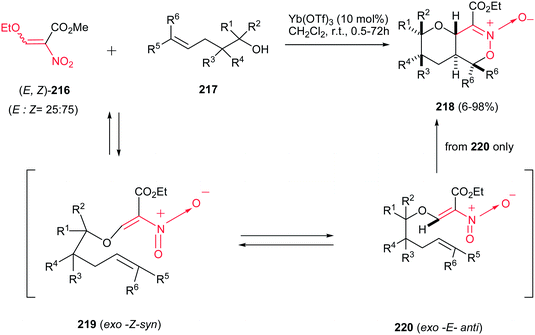
In another report, an efficient stereoselective tandem transetherification–intramolecular hetero Diels–Alder (HDA) reaction of (E,Z)-mixture of ethyl 2-nitro-3-ethoxyacrylate (E,Z)-216 and δ,ε-unsaturated alcohols 217 was reported by Wada et al. to achieve the functionalized bicyclic nitronates 218 (Scheme 62).142 The reaction proceeds with a catalytic amount of a Lewis acid such as Yb(OTf)3 under thermal condition in high yields. The stereochemistry of products is the result of the configurational control of transetherified intermediates under a rapid and reversible transetherification reaction pathway and affords trans-fused cyclic nitronates 218 as single stereoisomers in intramolecular HDA reaction.
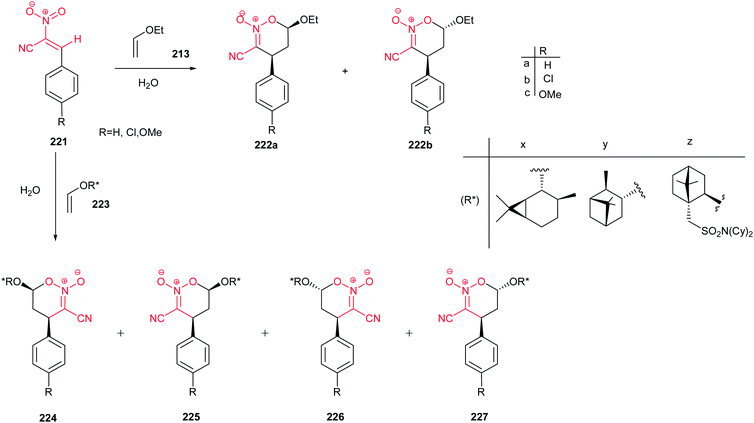
Fringuelli et al. demonstrated that the cycloaddition reactions of (E)-2-aryl-1-cyano-1-nitroalkenes 221 with ethyl vinyl ether 213 (Scheme 63) occur rapidly in water and are totally regio- and endo-selective toward synthesis of cyclic six-membered nitronates 222a and 222b.143
 | ||
| Scheme 63 Cycloaddition reactions of (E)-2-aryl-1-cyano-1-nitroalkenes 221 with ethyl vinyl ether 213 and 223. | ||
The asymmetric version of this reaction in pure water were explored by using enantiopure vinyl ethers (+)-223x, (+)-223y and (2)-223z. The results are summarized in Scheme 63 and Table 1. Excellent results were obtained by using chiral ethyl vinyl ether 223z derived from N,N-dicyclohexyl-(1S)-isoborneol-10-sulfonamide. Only optically active 224 and 226 cycloadducts were achieved which reflects a 100% asymmetric generation of 4R diasteroisomers.
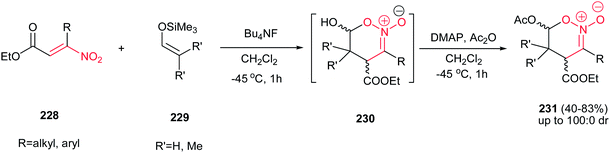
Finally, reactions of β-nitroacrylates 228 with silyl enol ethers 229 were investigated by Ballini et al. in 2009.144 They described that while silyl enol ethers derived from ketones afforded the corresponding Michael adducts in the presence of Bu4NF at −45 °C, silyl enol ethers arising from aldehydes provided the highly substituted hexahydro-4H-benzoxazine-2-oxides 231 under similar conditions in high yields and diastereoselectivities (Scheme 64). It is notable that intermediate 230 is unstable during the isolation process, and this problem was circumvented by the in situ acetylation of the hydroxyl group in the presence of DMAP and acetic anhydride.
6. Conclusions
Synthesis of heterocycles, often with high degree of selectivities, can be conveniently achieved using conjugated nitroalkenes as the key substrates. Nitroalkenes with a variety of substituents at α and β-positions and those that are part of a ring, are commonly employed for such purpose. The potential of nitroalkenes to take part in multi-component and cascade reactions, particularly, in diastereo- and enantioselective versions, has been demonstrated by research groups around the world. The reasons for the emergence of nitroalkenes as the sought after substrates lie primarily in the strong electron withdrawing and the co-ordinating capacity of the nitro group. The amenability of nitro group to undergo facile functional group conversion and high abundance of a wide variety of nitroalkenes have also inspired the organic chemists to employ nitroalkenes as their favorite substrates. In this scenario, the dominance of nitroalkenes as the model substrates in heterocycle synthesis will definitely continue as long as our quest for novel functionalized and fused heterocycles continues.Abbreviations
| Ac2O | Acetic anhydride |
| Ar | Aryl |
| Boc | tert-Butoxy carbamate |
| Bn | Benzyl |
| Cat. | Catalyst |
| Cbz | Benzyloxy carbamate |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DCE | Dichloroethane |
| DDQ | 2,3-Dichloro-5,6-dicyanobenzoquinone |
| DKR | Dynamic kinetic resolution |
| DMAP | 4-Dimethylaminopyridine |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| Dr | Diastereomeric ratio |
| Ee | Enantiomeric excess |
| Ent | Enantiomer |
| Epi | Epimer |
| HDA | Hetero Diels–Alder |
| i-Pr | isopropyl |
| LiHMDS | Lithium hexamethyldisilazide |
| MeOH | Methanol |
| MW | Microwave |
| NHC | N-Heterocyclic carbene |
| NMM | N-Methyl morpholine |
| NMO | N-Methylmorpholine-N-oxide |
| NOESY | Nuclear Overhauser effect spectroscopy |
| NOE | Nuclear Overhauser effect |
| Ns | 2-Nitrophenylsulfonyl |
| Ph | Phenyl |
| 1,10-Phen | 1,10-Phenanthroline |
| PPA | Polyphosphoric acid |
| PTC | Phase transfer catalyst |
| PTSA | p-Toluenesulfonic acid |
| Rt | Room temperature |
| TBAB | Tetrabutylammonium bromide |
| TCNiE | 1,1,2-Trichloro-2-nitroethene |
| TEAB | Tetraethylammonium bromide |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| THOs | Tetrahydro-1,2-oxazines |
| THPs | Tetrahydropyrans |
| TMS | Trimethylsilyl |
| TPAP | Tetrapropylammonium perruthenate |
| Ts | Tosyl |
Acknowledgements
Azim Ziyaei Halimehjani and Seyyed Emad Hooshmand thank the Faculty of Chemistry of Kharazmi University for supporting their research. Also, INNN thanks Department of Science and Technology (DST), India, for supporting research in the area of nitroalkene chemistry.References
- (a) M. J. Schneider, in Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Wiley, New York, 1996, vol. 10, ch. 3, p. 155 Search PubMed; (b) P. S. Watson, B. Jiang and B. Scott, Org. Lett., 2000, 2, 3679 CrossRef CAS PubMed; (c) S. Kallstrom and R. Leino, Bioorg. Med. Chem., 2008, 16, 601 CrossRef PubMed; (d) T. Yamashita, K. Yasuda, H. Kizu, Y. Kameda, A. A. Watson, R. J. Nash, G. W. J. Fleet and N. Asano, J. Nat. Prod., 2002, 65, 1875 CrossRef CAS PubMed; (e) A. Mochizuki, Y. Nakamoto, H. Naito, K. Uoto and T. Ohta, Bioorg. Med. Chem. Lett., 2008, 18, 782 CrossRef CAS PubMed.
- (a) P. E. Finke, B. Oates, S. G. Mills, M. MacCoss, L. Malkowitz, M. S. Springer, S. L. Gould, J. A. Demartino, A. Carella, G. Carver, K. Holmes, R. Danzeisen, D. Hazuda, J. Kessler, J. Lineberger, M. Miller, W. A. Schleif and E. A. Emini, Bioorg. Med. Chem. Lett., 2001, 11, 2475 CrossRef CAS PubMed; (b) A. A. Trabaco, N. Aerts, R. M. Alvarez, J. I. Andres, I. Boeckx, J. Fernandez, A. Gomez, F. E. Janssens, J. E. Leenaerts, A. I. D. Lucas, E. Matesanz, T. Steckler and S. Pullan, Bioorg. Med. Chem. Lett., 2007, 17, 3860 CrossRef PubMed; (c) J. Kobayashi and M. Ishibashi, Heterocycles, 1996, 42, 943 CrossRef CAS; (d) I. Ninomiya, T. Kiguchi and T. Naito, Alkaloids, 1998, 50, 317 CAS; (e) K. Murata, F. Takano, S. Fushiya and Y. Oshima, J. Nat. Prod., 1998, 61, 729 CrossRef CAS PubMed; (f) S. Kobayashi, M. Ueno, R. Suzuki, H. Ishitani, H. S. Kim and Y. Wataya, J. Org. Chem., 1999, 64, 6833 CrossRef CAS PubMed; (g) Y. Takaya, H. Tasaka, T. Chiba, K. Uwai, M. Tanitsu, H. S. Kim, Y. Wataya, M. Miura, M. Takeshita and Y. Oshima, J. Med. Chem., 1999, 42, 3163 CrossRef CAS PubMed; (h) R. F. Boswell, W. J. Welstead, R. L. Duncan, D. N. Johnson and W. H. Funderburk, J. Med. Chem., 1978, 21, 136 CrossRef PubMed.
- (a) P. D. Bailey, P. A. Millwood and P. D. Smith, Chem. Commun., 1998, 633 RSC; (b) Y. Tamaru, S.-I. Kawamura, T. Bando, K. Tanaka, M. Hojo and Z.-I. Yoshida, J. Org. Chem., 1988, 53, 5491 CrossRef CAS; (c) E. Lorthiois, I. Marek and J. F. Normant, J. Org. Chem., 1998, 63, 566 CrossRef CAS PubMed; (d) H. Poerwono, K. Higashiyama and H. Takahashi, J. Org. Chem., 1998, 63, 2711 CrossRef CAS PubMed; (e) S. Freville, M. Bonin, J. P. Celerier, H.-P. Husson, G. Lhommet, J. C. Quirion and V. M. Thuy, Tetrahedron, 1997, 53, 8447 CrossRef CAS.
- (a) K. A. Jørgensen, Angew. Chem., Int. Ed., 2000, 39, 3558 CrossRef; (b) P. Buonora, J. C. Olsen and T. Oh, Tetrahedron, 2001, 57, 6099 CrossRef CAS; (c) S. Kobayashi, in Cycloaddition Reaction in Organic Synthesis, ed. S. Kobayashi and K. A. Jørgensen, Wiley-VCH, Weinheim, 2002, p. 187, For selected examples, see Search PubMed; (d) D. L. Boger, W. L. Corbett and J. M. Wiggins, J. Org. Chem., 1990, 55, 2999 CrossRef CAS; (e) M. Teng and F. W. Fowler, J. Org. Chem., 1990, 55, 5646 CrossRef CAS; (f) D. L. Boger, W. L. Corbett, T. T. Curran and A. M. Kasper, J. Am. Chem. Soc., 1991, 113, 1713 CrossRef CAS; (g) C. Trione, L. M. Toledo, S. D. Kuduk, F. W. Fowler and D. S. Grierson, J. Org. Chem., 1993, 58, 2075 CrossRef CAS; (h) N. J. Sisti, I. A. Motorina, M. T. H. Dau, C. Riche, F. W. Fowler and D. S. Grierson, J. Org. Chem., 1996, 61, 3715 CrossRef CAS PubMed.
- (a) J. P. A. Harrity and O. Provoost, Org. Biomol. Chem., 2005, 3, 1349 RSC; (b) R. P. Hsung, A. V. Kurdyumov and N. Sydorenko, Eur. J. Org. Chem., 2005, 23 CrossRef CAS.
- (a) M. G. P. Buffat, Tetrahedron, 2004, 60, 1701 CrossRef CAS; (b) N. Sarkar, A. Banerjee and S. G. Nelson, J. Am. Chem. Soc., 2008, 130, 9222 CrossRef CAS PubMed; (c) S. E. Denmark and R. Y. Baiazitov, J. Org. Chem., 2006, 71, 593 CrossRef CAS PubMed; (d) T. Kobayashi, M. Nakashima, T. Hakogi, K. Tanaka and S. Katsumur, Org. Lett., 2006, 8, 3809 CrossRef CAS PubMed.
- (a) J. Liu, S. Lin, H. Ding, Y. Wei and F. Liang, Tetrahedron Lett., 2010, 51, 6349 CrossRef CAS; (b) S. Mishra and R. Ghosh, Tetrahedron Lett., 2011, 52, 2857 CrossRef CAS; (c) G. Brahmachari and S. Das, Tetrahedron Lett., 2012, 53, 1479 CrossRef CAS; (d) S. Cihalova, G. Valero, J. Schimer, M. Humpl, M. Dracinsky, A. Moyano, R. Rios and J. Vesely, Tetrahedron, 2011, 67, 8942 CrossRef CAS; (e) H. Zheng, X. Huo, C. Zhao, P. Jing, J. Yang, B. Fang and X. She, Org. Lett., 2011, 13, 6448 CrossRef CAS PubMed.
- Y. Chen, C. Zhong, J. L. Petersen, N. G. Akhmedov and X. Shi, Org. Lett., 2009, 11, 2333 CrossRef CAS PubMed.
- Y. Wang, S. Zhu and D. Ma, Org. Lett., 2011, 13, 1602 CrossRef CAS PubMed.
- T. Urushima, D. Sakamoto, H. Ishikawa and Y. Hayashi, Org. Lett., 2010, 12, 4588 CrossRef CAS PubMed.
- Y. Wang, D.-F. Yu, Y.-Z. Liu, H. Wei, Y.-C. Luo, D. J. Dixon and P.-F. Xu, Chem.–Eur. J., 2010, 16, 3922 CrossRef CAS PubMed.
- (a) J. Zakrzewski and M. Krawczyk, Heteroat. Chem., 2006, 17, 393 CrossRef CAS; (b) J. Zakrzewski and M. Krawczyk, Heteroat. Chem., 2008, 19, 549 CrossRef CAS; (c) H. Kizuka and D. R. Elmaleh, Nucl. Med. Biol., 1993, 20, 239 CrossRef CAS PubMed; (d) N. A. Kraus, Synthesis, 1973, 361 CrossRef; (e) R. Labas, G. Gilbert, O. Nicole, M. Dhilly, A. Abbas, O. Tirel, A. Buisson, J. Henry, L. Barré, D. Debruyne and F. Sobrio, Eur. J. Med. Chem., 2011, 46, 2295 CrossRef CAS PubMed.
- R. Chawla, A. Rai, A. K. Singh and L. D. S. Yadav, Tetrahedron Lett., 2012, 53, 5323 CrossRef CAS.
- H. Liu, Z. Zhou, Q. Sun, Y. Li, Y. Li, J. Liu, P. Yan, D. Wang and C. Wang, ACS Comb. Sci., 2012, 14, 366 CrossRef CAS PubMed.
- Z. Zhou, H. Liu, Q. Sun, Y. Li, J. Yang, J. Liu, P. Yan and C. Wang, Synlett, 2012, 2255 CAS.
- P. Jakubec, M. Helliwell and D. J. Dixon, Org. Lett., 2008, 10, 4267 CrossRef CAS PubMed.
- M. Balasubramanian and J. G. Keay, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. V. F. Scriven, Pergamon Press, London, 1996, vol. 5, ch. 6, p. 245 Search PubMed.
- (a) B. Y. Kim, J. B. Ahn, H. W. Lee, S. K. Kang, J. H. Lee, J. S. Shin, S. K. Ahn, C. I. Hong and S. S. Yoon, Eur. J. Med. Chem., 2004, 39, 433 CrossRef CAS PubMed; (b) I. J. Enyedy, S. Sakamuri, W. A. Zaman, K. M. Johnson and S. Wang, Bioorg. Med. Chem. Lett., 2003, 13, 513 CrossRef CAS PubMed; (c) A. D. Pillai, P. D. Rathod, P. X. Franklin, M. Patel, M. Nivsarkar, K. K. Vasu, H. Padh and V. Sudarsanam, Biochem. Biophys. Res. Commun., 2003, 301, 183 CrossRef CAS PubMed; (d) V. Klimešová, M. Svoboda, K. Waisser, M. Pour and J. Kaustová, Farmaco, 1999, 54, 666 CrossRef; (e) B. B. Fredholm, A. P. Ijzerman, K. A. Jacobson, K.-N. Klotz and J. Linden, Pharmacol. Rev., 2001, 53, 527 CAS; (f) S. B. Levy, M. N. Alekshun, B. L. Podlogar, K. Ohemeng, A. K. Verma, T. Warchol, B. Bhatia, T. Boowser and M. U. S. Grier, Pat. Appl., US 2005124678, A1, 2005; (g) H. Harada, S. Watanuki, T. Takuwa, K. Kawaguchi, T. Okazaki, Y. Hirano and C. Saitoh, PCT Int. Appl., WO 2002006237 Al, 2002; (h) L. C. W. Chang, J. K. Von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. F. Spanjersberg, S. F. Roerink, G. van den Hout, M. W. Beukers, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2005, 48, 2045 CrossRef CAS PubMed; (i) M. W. Beukers, L. C. W. Chang, J. K. Von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. F. Spanjersberg, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2004, 47, 3707 CrossRef CAS PubMed.
- (a) F. R. Buhler and W. Kiowski, J. Hypertens., 1987, S3, 5 Search PubMed; (b) J. L. Reid, P. A. Meredith and F. Pasanisi, J. Cardiovasc. Pharmacol., 1985, S18, 7 Search PubMed; (c) R. A. Janis, P. J. Silver and D. J. Triggle, Adv. Drug Res., 1987, 16, 309 CAS.
- (a) Y.-F. Wang and S. Chiba, J. Am. Chem. Soc., 2009, 131, 12570 CrossRef CAS PubMed; (b) Y. Wei and N. Yoshikai, J. Am. Chem. Soc., 2013, 135, 3756 CrossRef CAS PubMed; (c) M. D. Fletcher, T. E. Hurst, T. J. Miles and C. J. Moody, Tetrahedron, 2006, 62, 5454 CrossRef CAS; (d) N. M. Evdokimov, I. V. Magedov, A. S. Kireev and A. Kornienko, Org. Lett., 2006, 8, 899 CrossRef CAS PubMed; (e) M. Movassaghi and M. D. Hill, J. Am. Chem. Soc., 2006, 128, 4592 CrossRef CAS PubMed; (f) K. Tanaka, H. Mori, M. Yamamoto and S. Katsumara, J. Org. Chem., 2001, 66, 3099 CrossRef CAS PubMed; (g) A. R. Renslo and R. L. Danheiser, J. Org. Chem., 1998, 63, 7840 CrossRef CAS; (h) M. D. Hill, Chem.–Eur. J., 2010, 16, 12052 CrossRef CAS PubMed; (i) V. V. Lipson and N. Y. Gorobets, Mol. Diversity, 2009, 13, 399 CrossRef CAS PubMed; (j) J. A. Varela and C. Saá, Chem. Rev., 2003, 103, 3787 CrossRef CAS PubMed.
- (a) A. Hantzsch, Liebigs Ann. Chem., 1882, 215, 1 CrossRef; (b) B. Guillaume and A. B. Charette, J. Am. Chem. Soc., 2008, 130, 18 CrossRef PubMed.
- T. R. Kelly and R. L. Lebedev, J. Org. Chem., 2002, 67, 2197 CrossRef CAS PubMed.
- (a) B. C. Ranu, R. Jana and S. Sowmiah, J. Org. Chem., 2007, 72, 3152 CrossRef CAS PubMed; (b) K. Guo, M. J. Thompson, T. R. K. Reddy, R. Mutter and B. Chen, Tetrahedron, 2007, 63, 5300 CrossRef CAS; (c) K. Guo, M. J. Thompson and B. Chen, J. Org. Chem., 2009, 74, 6999 CrossRef CAS PubMed; (d) B. Das, B. Ravikanth, A. S. Kumar and B. S. Kanth, J. Heterocycl. Chem., 2009, 46, 1208 CrossRef CAS.
- (a) C. Mukhopadhyay, P. K. Tapaswi and R. J. Butcher, Tetrahedron Lett., 2010, 51, 1797 CrossRef CAS; (b) L. Nagarapu, Aneesa, R. Peddiraju and S. Apuri, Catal. Commun., 2007, 8, 1973 CrossRef CAS; (c) M. Adib, H. Tahermansouri, S. A. Koloogani, B. Mohammadi and H. R. Bijanzadeh, Tetrahedron Lett., 2006, 47, 5957 CrossRef CAS.
- D. L. Boger, J. S. Panek and M. M. Meier, J. Org. Chem., 1982, 47, 895 CrossRef CAS.
- (a) X. Xiong, M. C. Bagley and K. Chapaneri, Tetrahedron Lett., 2004, 45, 6121 CrossRef CAS; (b) M. C. Bagley, J. W. Dale and J. Bower, Synlett, 2001, 1149 CrossRef CAS.
- V. O. Iaroshenko, S. Mkrtchyan, A. Gevorgyan, M. Vilches-Herrera, D. V. Sevenard, A. Villinger, T. V. Ghochikyan, A. Saghiyan, V. Y. Sosnovskikh and P. Langer, Tetrahedron, 2012, 68, 2532 CrossRef CAS.
- V. Y. Korotaev, A. Y. Barkov and V. Y. Sosnovskikh, Tetrahedron Lett., 2013, 54, 3091 CrossRef CAS.
- A. Diaz-Ortiz, J. R. Carrillo, F. P. Cossio, M. J. Gomez-Escalonilla, A. DelaHoz, A. Moreno and P. Prieto, Tetrahedron, 2000, 56, 1569 CrossRef CAS.
- A. Alizadeh, A. Mikaeili and T. Firuzyar, Synthesis, 2012, 1380 CrossRef CAS.
- A. Alizadeh, T. Firuzyar and A. Mikaeili, Synthesis, 2010, 3913 CrossRef CAS.
- A. Alizadeh and A. Rezvanian, Synlett, 2011, 1105 CrossRef CAS.
- M. M. Sarmah, D. Prajapati and W. Hu, Synlett, 2013, 471 CAS.
- (a) J. A. Eussell, Annu. Rev. Biochem., 1945, 14, 309 CrossRef; (b) R. A. Cox, Q. Rev., Chem. Soc., 1968, 22, 499 RSC.
- (a) K. Desai, R. Patel and K. Chikhalia, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2006, 45, 773 Search PubMed; (b) A. E. Amr, M. S. Nermien and M. M. Abdulla, Monatsh. Chem., 2007, 138, 699 CrossRef CAS; (c) N. Fujiwara, T. Nakajima, Y. Ueda, H. Fujita and H. Kawakami, Bioorg. Med. Chem., 2008, 16, 9804 CrossRef CAS PubMed; (d) L. Ballell, R. A. Field, G. A. C. Chung and R. J. Young, Bioorg. Med. Chem. Lett., 2007, 17, 1736 CrossRef CAS PubMed; (e) E. Wagner, K. Al-Kadasi, M. Zimecki and W. Sawka-Dobrowolska, Eur. J. Med. Chem., 2008, 43, 2498 CrossRef CAS PubMed; (f) L. Cordeu, E. Cubedo, E. Bandres, A. Rebollo, X. Saenz, H. Chozas, M. Victoria Dominguez, M. Echeverria, B. Mendivil and C. Sanmartin, Bioorg. Med. Chem., 2007, 15, 1659 CrossRef CAS PubMed; (g) K. Gorlitzer, S. Herbig and R. D. Walter, Pharmazie, 1997, 52, 670 CAS; (h) I. V. Ukrainets, I. A. Tugaibei, N. L. Bereznykova, V. N. Karvechenko and A. V. Turov, Chem. Heterocycl. Compd., 2008, 5, 565 CrossRef; (i) M. Kurono, M. Hayashi, K. Miura, Y. Isogawa and K. Sawai, Kokai Tokkyo Koho JP 62, 267, 272, Sanwa Kagaku Kenkyusho Co., Japan, 1987, Chem. Abstr., 1988, 109, 37832t.
- V. A. Zapol'skii, J. C. Namyslo, C. Altug, M. Gjikaj and D. E. Kaufmann, Synthesis, 2008, 304 Search PubMed.
- S. Gabrielli, R. Ballini and A. Palmieri, Monatsh. Chem., 2013, 144, 509 CrossRef CAS.
- (a) A. Kumar, K. Srivastava, S. R. Kumar, S. K. Puri and P. M. S. Chauhan, Bioorg. Med. Chem. Lett., 2008, 18, 6530 CrossRef CAS PubMed; (b) X. Ma, W. Zhou and R. Brun, Bioorg. Med. Chem. Lett., 2009, 19, 986 CrossRef CAS PubMed; (c) A. R. Gholap, K. S. Toti, F. Shirazi, R. Kumari, M. K. Bhat, M. V. Deshpande and K. V. Srinivasan, Bioorg. Med. Chem., 2007, 15, 6705 CrossRef CAS PubMed; (d) S. Rossiter, S. J. Peron, P. J. Whitfield and K. Jones, Bioorg. Med. Chem. Lett., 2005, 15, 4806 CrossRef CAS PubMed; (e) K. Atwal, Eur. Pat., EP 488,616, 1992, Chem. Abstr., 1992, 117, 89978e; (f) R. W. Carling, P. D. Leeson, A. M. Moseley, J. D. Smith, K. Saywell, M. D. Triclehank, J. A. Kemp, G. R. Marshall, A. C. Foster and S. Grimwood, Bioorg. Med. Chem. Lett., 1993, 3, 65 CrossRef CAS; (g) J. K. Barbay, Y. Gong, M. Buntinx, J. Li, C. Claes, P. J. Hornby, G. Van Lommen, J. VanWauwe and W. He, Bioorg. Med. Chem. Lett., 2008, 18, 2544 CrossRef CAS PubMed; (h) G. Dorey, B. Lockhart, P. Lestage and P. Casara, Bioorg. Med. Chem. Lett., 2000, 10, 935 CrossRef CAS PubMed; (i) I. Berenguer, N. El Aouad, S. Andujar, V. Romero, F. Suvire, T. Freret, A. Bermejo, M. D. Ivorra, R. D. Enriz, M. Boulouard, N. Cabedo and D. Cortes, Bioorg. Med. Chem., 2009, 17, 4968 CrossRef CAS PubMed; (j) A. H. Abadi, G. H. Hegazy and A. A. E. Zaher, Bioorg. Med. Chem., 2005, 13, 5759 CrossRef CAS PubMed.
- (a) J. L. Kim, I. S. Shin and H. Kim, J. Am. Chem. Soc., 2005, 127, 1614 CrossRef CAS PubMed; (b) A. K. Agrawal and S. A. Jenekhe, Chem. Mater., 1996, 8, 579 CrossRef CAS; (c) S. A. Jenekhe, L. Lu and M. M. Alam, Macromolecules, 2001, 34, 7315 CrossRef CAS; (d) S. Majumder, K. R. Gipson and A. Odom, Org. Lett., 2009, 11, 4720 CrossRef CAS PubMed.
- (a) C. S. Cho, B. H. Oh and S. C. Shim, Tetrahedron Lett., 1999, 40, 1499 CrossRef CAS; (b) L. Zhou and Y. Zhang, J. Chem. Soc., Perkin Trans. 1, 1998, 2899 RSC; (c) R. C. Larock and M.-Y. Kero, Tetrahedron Lett., 1991, 32, 569 CrossRef CAS; (d) L. Zhou, S. Tu, D. Shi, G. Dai and W. Chen, Synthesis, 1988, 851 Search PubMed; (e) R. C. Larock and S. Babu, Tetrahedron Lett., 1987, 28, 5291 CrossRef CAS; (f) F. Ozawa, H. Yanagihara and A. Yamamoto, J. Org. Chem., 1986, 51, 415 CrossRef CAS; (g) G. Jones, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, New York, 1996, vol. 5, p. 167 Search PubMed; (h) C. S. Cho, B. H. Oh, T. J. Kim, T. J. Kim and S. C. Shim, Chem. Commun., 2000, 1885 RSC; (i) B. Jiang and Y. G. Si, J. Org. Chem., 2002, 67, 9449 CrossRef CAS PubMed; (j) H. Skraup, Chem. Ber., 1880, 13, 2086 Search PubMed; (k) P. Friedlander, Chem. Ber., 1882, 15, 2572 CrossRef; (l) R. H. Mansake and M. Kulka, Org. React., 1953, 7, 59 Search PubMed; (m) R. J. Linderman and K. S. Kirollos, Tetrahedron Lett., 1990, 31, 2689 CrossRef CAS; (n) M. E. Theoclitou and L. A. Robinson, Tetrahedron Lett., 2002, 43, 3907 CrossRef CAS; (o) N. T. Patil and V. S. Raut, J. Org. Chem., 2010, 75, 6961 CrossRef CAS PubMed; (p) R. P. Korivi and C.-H. Cheng, J. Org. Chem., 2006, 71, 7079 CrossRef CAS PubMed; (q) S. P. Fearley and C. Thongsornkleeb, J. Org. Chem., 2010, 75, 933 CrossRef PubMed; (r) T. Akiyama, H. Morita and K. Fuchibe, J. Am. Chem. Soc., 2006, 128, 13070 CrossRef CAS PubMed; (s) H. Liu, G. Dagousset, G. Masson, P. Retailleau and J. Zhu, J. Am. Chem. Soc., 2008, 131, 4598 CrossRef PubMed; (t) L. He, M. Bekkaye, P. Retailleau and G. Masson, Org. Lett., 2012, 14, 3158 CrossRef CAS PubMed; (u) G. Dagousset, J. Zhu and G. Masson, J. Am. Chem. Soc., 2011, 133, 14804 CrossRef CAS PubMed; (v) L. S. Povarov, V. I. Grigos and B. M. Mikhailov, Izv. Akad. Nauk. SSR, Ser. Khim., 1963, 2039 CAS.
- (a) X. Li, C. Li, W. Zhang, X. Lu, S. Han and R. Hong, Org. Lett., 2010, 12, 1696 CrossRef CAS PubMed; (b) A.-M. L. Hogan and D. F. O'Shea, Org. Lett., 2006, 8, 3769 CrossRef CAS PubMed; (c) B. V. Subba Reddy, D. Medaboina, B. Sridhar and K. K. Singarapu, J. Org. Chem., 2013, 78, 8161 CrossRef CAS PubMed.
- Z.-H. Yu, H.-F. Zheng, W. Yuan, Z.-L. Tang, A.-D. Zhang and D.-Q. Shi, Tetrahedron, 2013, 69, 8137 CrossRef CAS.
- M. C. Yan, Z. Tu, C. Lin, S. Ko, J. Hsu and C.-F. Yao, J. Org. Chem., 2004, 69, 1565 CrossRef CAS PubMed.
- R. Ballini, G. Bosica, D. Fiorini and A. Palm, Green Chem., 2005, 7, 825 RSC.
- A. V. Aksenov, A. N. Smirnov, N. A. Aksenov, I. V. Aksenova, L. V. Frolova, A. Kornienko, I. V. Magedovz and M. Rubin, Chem. Commun., 2013, 49, 9305 RSC.
- A. Rai, A. K. Singh, P. Singh and L. D. S. Yadav, Tetrahedron Lett., 2011, 52, 1354 CrossRef CAS.
- A. Rai, A. K. Singh, S. Singh and L. D. S. Yadav, Synthesis, 2011, 335 CAS.
- K. Balamurugan, V. Jeyachandran, S. Perumal and J. C. Menendez, Tetrahedron, 2011, 67, 1432 CrossRef CAS.
- (a) P. R. Moore, A. Evenson, T. D. Luckey, E. McCoy, C. A. Elvehjem and E. B. Hart, J. Biol. Chem., 1946, 165, 437 CAS; (b) M. G. Moloney, Nat. Prod. Rep., 2002, 19, 597 RSC.
- D. Rose, E. Lieske, H. Hoeffkes and K.-G. Henkel, German Offen., DE 3, 825, 212, 1990, Chem. Abstr., 1988, 109, 73473.
- J. B. Rangisetty, C. N. V. H. B. Gupta, A. L. Prasad, N. Sridhar, P. Parimoo and A. Veeranjaneyulu, J. Pharm. Pharmacol., 2001, 53, 1409 CrossRef CAS PubMed.
- M. M. Badran, K. A. M. Abouzid and M. H. M. Hussein, Arch. Pharmacal Res., 2003, 26, 107 CrossRef CAS.
- (a) A. Carta, P. Corona and M. Loriga, Curr. Med. Chem., 2005, 12, 2259 CrossRef CAS PubMed; (b) A. Carta, G. Paglietti, M. E. R. Nikookar, P. Sanna, L. Sechi and S. Zanetti, Eur. J. Med. Chem., 2002, 37, 355 CrossRef CAS PubMed; (c) A. Jaso, B. Zarranz, I. Aldana and A. Monge, Eur. J. Med. Chem., 2003, 38, 791 CrossRef CAS PubMed; (d) U. Das, H. N. Pati, A. K. Panda, E. De Clercq, J. Balzarini, J. Molnár, Z. Baráth, I. Ocsovszki, M. Kawase, L. Zhou, H. Sakagami and J. R. Dimmock, Bioorg. Med. Chem., 2009, 17, 3909 CrossRef CAS PubMed.
- A. Carta, P. Sanna, L. Gherardini and S. Zanetti, II Farmaco, 2001, 56, 933 CrossRef CAS.
- R. Sarges, H. R. Howard, R. G. Browne, L. A. Lebel, A. Seymour and B. K. Koe, J. Med. Chem., 1990, 33, 2240 CrossRef CAS PubMed.
- (a) C. Bailly, S. Echepare, F. Gago and M. Waring, Anti-Cancer Drug Des., 1999, 14, 291 CAS; (b) S. A. Raw, C. D. Wilfred and R. J. K. Taylor, Chem. Commun., 2003, 2286 RSC.
- U. J. Ries, H. W. Priekpe, N. H. Hauel, S. Handschuh, G. Mihm, J. M. Stassen, W. Wienen and H. Nar, Bioorg. Med. Chem. Lett., 2003, 13, 2297 CrossRef CAS PubMed.
- D. S. Su and M. G. Bock, US Pat., 20050020591, 2005.
- (a) J. A. Butera, W. Spinelli, V. Anantharaman, N. Marcopulos, R. W. Parsons, I. F. Moubarak, C. Cullinan and J. F. Bagli, J. Med. Chem., 1991, 34, 3212 CrossRef CAS PubMed; (b) R. Aguilera, J. C. Duplant and C. Nofre, Bull. Soc. Chim. Fr., 1968, 11, 4491 Search PubMed; (c) W. L. F. Armarego, P. Waring and B. Paal, Aust. J. Chem., 1982, 35, 785 CrossRef CAS; (d) G. H. Fisher and H. P. Schultz, J. Org. Chem., 1974, 39, 635 CrossRef CAS; (e) M. Battistini, E. Erba and D. Pocar, J. Chem. Soc., Perkin Trans. 1, 1993, 339 RSC; (f) S. Matsuura, T. Sugimoto, H. Haegawa, S. Imaizumi and A. Ichiyama, J. Biochem., 1980, 87, 951 CAS.
- Z. Jones, R. Groneberg and M. Drew and C. T. Eary, US Pat., 20050282812, 2005.
- (a) H. Takaya, T. Ohta and R. Noyori, in Catalytic Asymmetric Synthesis,ed. I. Ojima, VCH, New York, 1993, p. 31 Search PubMed; (b) B. R. James, Catal. Today, 1997, 37, 209 CrossRef CAS.
- H. Suschitzky, B. J. Wakefield and R. A. Whittaker, J. Chem. Soc., Perkin Trans. 1, 1975, 401 RSC.
- See, for example: (a) K. S. Kim, L. Qian, J. E. Bird, K. E. J. Dickinson, S. Moreland, T. R. Schaeffer, T. L. Waldron, C. L. Delaney, H. N. Weller and A. V. Miller, J. Med. Chem., 1993, 36, 2335 CrossRef CAS PubMed; (b) Y. Kim, M. H. Lee, E. T. Choi, E. S. No and Y. S. Park, Heterocycles, 2007, 71, 5 CrossRef CAS; (c) S. Kamila and E. R. Biehl, Heterocycles, 2006, 68, 1931 CrossRef CAS; (d) Y. Kim, K. H. Kang, E. T. Choi, M. H. Lee and Y. S. Park, Bull. Korean Chem. Soc., 2007, 28, 325 CrossRef CAS.
- T. M. A. El-Maati, Bull. Chim. Farm., 1999, 138, 272 CAS.
- (a) F. Juncai, L. Yang, M. Qinghua and L. Bin, Synth. Commun., 1998, 28, 193 CrossRef; (b) J. Barluenga, F. Aznar, R. Liz and M. P. Cabal, Synthesis, 1985, 313 CrossRef CAS; (c) T. Eicher and S. Hauptmann, The Chemistry of Heterocycles, Thieme, New York, 1995, p. 417 Search PubMed; (d) P. A. Petukhov and A. V. Tkachev, Tetrahedron, 1997, 53, 9761 CrossRef CAS; (e) D. J. Brown, in The Chemistry of Heterocyclic Compounds, ed. E. C. Taylor and P. Wipf, John Wiley & Sons, Hoboken, NJ, 2004 Search PubMed.
- S. Murata, T. Sugimoto and S. Matsuura, Heterocycles, 1987, 26, 883 CrossRef CAS.
- Y. Chen, K. Li, M. Zhao, Y. Li and B. Chen, Tetrahedron Lett., 2013, 54, 1627 CrossRef CAS.
- E. Merisor and U. Beifuss, Tetrahedron Lett., 2007, 48, 8383 CrossRef CAS.
- C. Meyer, V. A. Zapol'skii, A. E. W. Adam and D. E. Kaufmann, Synthesis, 2008, 2575 CAS.
- J. Maichrowski, M. Gjikaj, E. G. Hübner, B. Bergmann, I. B. Müller and D. E. Kaufmann, Eur. J. Org. Chem., 2013, 2091 CrossRef CAS.
- R. Ballini, S. Gabrielli and A. Palmieri, Synlett, 2009, 965, 209 Search PubMed.
- K. V. Kovalenko, S. V. Makarenko, K. V. Altukhov and V. M. Berestovitskaya, Russ. J. Gen. Chem., 2010, 80, 158 CrossRef CAS.
- (a) T. L. B. Boivin, Tetrahedron, 1987, 43, 3309 CrossRef CAS; (b) F. P. Marmster and F. G. West, Chem.–Eur. J., 2002, 8, 4347 Search PubMed; (c) M. T. Crimmins, J. L. Zuccarello, J. M. Ellis, P. J. McDougall, P. A. Haile, J. D. Parrish and K. A. Emmitte, Org. Lett., 2009, 11, 489 CrossRef CAS PubMed; (d) M. Schwarz, G. F. Graminski and R. M. Waters, J. Org. Chem., 1986, 51, 260 CrossRef CAS; (e) A. B. Smith III, R. J. Fox and T. M. Razler, Acc. Chem. Res., 2008, 41, 675 CrossRef PubMed; (f) K. Yasui, Y. Tamura, T. Nakatani, K. Kawada and M. Ohtani, J. Org. Chem., 1995, 60, 7567 CrossRef CAS; (g) V. Dumontet, N. Van Hung, M. T. Adeline, C. Riche, A. Chiaroni, T. Sévenet and F. Guéritte, J. Nat. Prod., 2004, 67, 858 CrossRef CAS PubMed; (h) Â. de Fátima, L. K. Kohn, J. E. de Carvalho and R. A. Pilli, Bioorg. Med. Chem., 2006, 14, 622 CrossRef PubMed; (i) A. O. M. Maria, O. Donadel, G. H. Wendel, J. A. Guzman, E. Guerreiro and O. S. Giordano, Biol. Pharm. Bull., 2000, 23, 555 CrossRef CAS PubMed; (j) Â. de Fátima, L. K. Kohn, M. A. Antônio, J. E. de Carvalho and R. A. Pilli, Bioorg. Med. Chem., 2004, 12, 5437 CrossRef PubMed; (k) M. Saeed, T. IIg, M. Schick, M. Abbas and W. Voelter, Tetrahedron Lett., 2001, 42, 7401 CrossRef CAS; (l) S. L. Capim, G. M. Gonçalves, G. C. M. dos Santos, B. G. Marinho and M. L. A. A. Vasconcellos, Bioorg. Med. Chem., 2013, 21, 6003 CrossRef CAS PubMed.
- (a) P. A. Clarke and S. Santo, Eur. J. Org. Chem., 2006, 2045 CrossRef CAS; (b) H. Fuwa, Hetereocycles, 2012, 85, 1255 CrossRef CAS; (c) H. Fuwa, N. Ichinokawa, K. Noto and M. Sasaki, J. Org. Chem., 2012, 77, 2588 CrossRef CAS PubMed; (d) S. Roscales and A. G. Csákÿ, Org. Lett., 2012, 14, 1187 CrossRef CAS PubMed.
- W. J. Morris, D. W. Custar and K. A. Scheidt, Org. Lett., 2005, 7, 1113 CrossRef CAS PubMed.
- A. B. Smith III, S. Dong, J. B. Brenneman and R. J. Fox, J. Am. Chem. Soc., 2009, 131, 12109 CrossRef PubMed.
- (a) L. F. Tietze, G. Kettschau, J. A. Gewert and A. Schuffenhauer, Curr. Org. Chem., 1998, 2, 19 CAS; (b) A. G. Dossetter, T. F. Jamison and E. N. Jacobsen, Angew. Chem., Int. Ed., 1999, 38, 2398 CrossRef CAS; (c) H. Du, X. Zhang, Z. Wang, H. Bao, T. You and K. Ding, Eur. J. Org. Chem., 2008, 2248 CrossRef CAS; (d) Y. Huang, A. K. Unni, A. N. Thadani and V. H. Rawal, Nature, 2003, 424, 146 CrossRef CAS PubMed.
- R. Jasti, J. Vitale and S. D. Rychnovsky, J. Am. Chem. Soc., 2004, 126, 9904 CrossRef CAS PubMed.
- F. Hilli, J. M. White and M. Rizzacasa, Org. Lett., 2004, 6, 1289 CrossRef CAS PubMed.
- I. Paterson, D. Y. K. Chen, M. J. Coster, J. L. Acena, J. Bach, K. R. Gibson, L. E. Keown, R. M. Oballa, T. Trieselmann, D. J. Wallace, A. P. Hodgson and R. D. Norcross, Angew. Chem., Int. Ed., 2001, 40, 4055 CrossRef CAS.
- (a) B. M. Trost, H. Yang and G. Wuitschik, Org. Lett., 2005, 7, 4761 CrossRef CAS PubMed; (b) B. M. Trost, M. R. B. Machacek and D. Faulk, J. Am. Chem. Soc., 2006, 128, 6745 CrossRef CAS PubMed.
- L. Wang, P. Li and D. Menche, Angew. Chem., Int. Ed., 2010, 49, 9270 CrossRef CAS PubMed; L. Wang and D. Menche, J. Org. Chem., 2012, 77, 10811 CrossRef PubMed.
- Y. Wu, A. Lu, Y. Liu, X. Yu, Y. Wang, G. Wu, H. Song, Z. Zhou and C. Tang, Tetrahedron: Asymmetry, 2010, 21, 2988 CrossRef CAS.
- A. Adibekian, M. S. M. Timmer, P. Stallforth, J. van Rijn, D. B. Werz and P. H. Seeberger, Chem. Commun., 2008, 3549 RSC.
- H. Uehara, R. Imashiro, G. Hernández-Torres and C. F. Barbas III, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20672 CrossRef CAS PubMed.
- R. Imashiro, H. Uehara and C. F. Barbas III, Org. Lett., 2010, 12, 5250 CrossRef CAS PubMed.
- X. Y. Chen, L. H. Sun and S. Ye, Chem.–Eur. J., 2013, 19, 4441 CrossRef CAS PubMed.
- (a) T. Kuwada, K. Harada, J. Nobuhiro and T. Choshi, Heterocycles, 2002, 57, 2081 CrossRef CAS; (b) E. E. Schweizer and O. Meeder-Nycz, Chromenes, Chromansses, and Chromones, Wiley-Interscience, New York, 1977 Search PubMed; (c) K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G. Q. Cao, S. Barluenga and H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939 CrossRef CAS; (d) G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285 CrossRef CAS PubMed; (e) B. M. Trost, H. C. Shen, L. Dong, J. P. Surivet and C. Sylvain, J. Am. Chem. Soc., 2004, 126, 11966 CrossRef CAS PubMed; (f) A. Afantitis, G. Melagraki, H. Sarimveis, P. A. Koutentis, J. Markopoulos and O. Igglessi-Markopoulou, Bioorg. Med. Chem., 2006, 14, 6686 CrossRef CAS PubMed; (g) C. Conti, L. P. Monaco and N. Desideri, Bioorg. Med. Chem., 2011, 19, 7357 CrossRef CAS PubMed; (h) D. M. Roll, J. K. Manning and G. T. Carter, J. Antibiot., 1998, 51, 635 CrossRef CAS PubMed; (i) J. M. Grisar, M. A. Petty, F. N. Bolkenius, J. Dow, J. Wagner, E. R. Wagner, K. D. Haegele and W. D. Jong, J. Med. Chem., 1991, 34, 257 CrossRef CAS PubMed.
- G. W. Burton and K. U. Ingold, Acc. Chem. Res., 1986, 19, 194 CrossRef CAS.
- (a) M. M. Khafagy, A. H. F. A. El-Wahas, F. A. Eid and A. M. El-Agrody, II Farmaco, 2002, 57, 715 CrossRef CAS; (b) W. P. Smith, L. S. Sollins, D. P. Howes, C. P. Cherry, D. I. Starkey and N. K. Cobley, J. Med. Chem., 1998, 41, 787 CrossRef PubMed; (c) A. G. Martinez and L. J. Marck, Bioorg. Med. Chem. Lett., 1997, 7, 3165 CrossRef; (d) C. P. Dell and C. W. Smith, EP Pat., 537949, 1993, Chem. Abstr., 1993, 119, 139102d; (e) G. Bianchi and A. Tava, Agric. Biol. Chem., 1987, 51, 2001 CrossRef CAS; (f) S. J. Mohr, M. A. Chirigios, F. S. Fuhrman and J. W. Pryor, Cancer Res., 1975, 35, 3750 CAS; (g) E. A. Kaczka, F. J. Wolf, F. P Rathe and K. J. Folkers, J. Am. Chem. Soc., 1955, 77, 6404 CrossRef CAS; (h) E. E. Smissman, C. O. Wilson, O. Gisvold and R. F. Doerge, Textbook of organic Medicinal and Pharmaceutical Chemistry, Lippincott Co., Philadelphia, Toronto, 8th edn, 1982, p. 291 Search PubMed; (i) D. R. Anderson, S. Hegde, E. Reinhard, L. Gomez, W. F. Vernier, L. Lee, S. Liu, A. Sambandam, P. A. Sinder and L. Masih, Bioorg. Med. Chem. Lett., 2005, 15, 1587 CrossRef CAS PubMed; (j) J. Skommer, D. Wlodkowic, M. Matto, M. Eray and J. Pelkonen, Leuk. Res., 2006, 30, 322 CrossRef CAS PubMed; (k) J. L. Wang, D. Liu, Z. Zhang, S. Shan, X. Han, S. M. Srinvasula, C. M. Croce, E. S. Alnemeri and Z. Huang, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 7124 CrossRef CAS PubMed; (l) F. Eiden and F. Denk, Arch. Pharm. Weinhein Ger., 1991, 324, 353 CrossRef CAS.
- N. Yu, J. M. Aramini, M. W. Germann and Z. Huang, Tetrahedron Lett., 2000, 41, 6993 CrossRef CAS.
- (a) K. C. Nicolaou, R. Reingruber, D. Sarlah and S. Brase, J. Am. Chem. Soc., 2009, 131, 2086 CrossRef CAS PubMed; (b) X. Wu, J. Zhou and B. B. Snider, Angew. Chem., Int. Ed., 2009, 48, 1283 CrossRef CAS PubMed; (c) S. Chang and R. H. Grubbs, J. Org. Chem., 1998, 63, 864 CrossRef CAS PubMed; (d) S. Gowrisankar, K. Y. Lee and J. N. Kim, Bull. Korean Chem. Soc., 2007, 28, 624 CrossRef CAS; (e) M. A. Ibrahim, T. E. Ali, Y. A. Alnamer and Y. A. Gabr, ARKIVOC, 2010, (i), 98 Search PubMed; (f) S. Khadem and R. J. Marles, Molecules, 2012, 17, 191 CrossRef CAS PubMed; (g) R. B. Patil, S. D. Sawant and P. A. Thombare, Int. J. PharmTech Res., 2012, 4, 375 CAS; (h) E. Corradini, P. Foglia, P. Giansanti, R. Gubbiotti, R. Samperi and A. Laganà, Nat. Prod. Res., 2011, 25, 469 CrossRef CAS PubMed; (i) A. K. Verma and R. Pratap, Tetrahedron, 2012, 68, 8523 CrossRef CAS; (j) J. H. Cha, Y. S. Cho, H. Y. Koh, E. Lee, Y. T. Kim, H. H. Yang and H. Y. Kang, Bull. Korean Chem. Soc., 2004, 25, 1123 CrossRef CAS; (k) J. I. Lee, H. S. Son and M. G. Jung, Bull. Korean Chem. Soc., 2005, 26, 1461 CrossRef CAS; (l) H. Miyazaki, Y. Honda, K. Honda and S. Inoue, Tetrahedron Lett., 2000, 41, 2643 CrossRef CAS; (m) N. A. Petasis and A. Butkevich, J. Organomet. Chem., 2009, 694, 1747 CrossRef CAS PubMed; (n) I. Rodriguez, S. Iborra, F. Rey and A. Corma, Appl. Catal., A, 2000, 194–195, 241 CrossRef CAS; (o) Q. Wang and M. G. Finn, Org. Lett., 2000, 2, 4063 CrossRef CAS PubMed; (p) J. S. Yadav, B. V. S. Reddy, L. Chandraiah, B. Jagannadh, S. K. Kumar and A. C. Kunwar, Tetrahedron Lett., 2002, 43, 4527 CrossRef CAS; (q) J. S. Yadav, B. V. S. Reddy, C. Parisse, P. Carvalho and T. P. Rao, Tetrahedron Lett., 2002, 43, 2999 CrossRef CAS; (r) J. S. Yadav, B. V. S. Reddy, M. Aruna, C. Venugopal, T. Ramalingam, S. K. Kumar and A. C. Kunwar, J. Chem. Soc., Perkin Trans. 1, 2002, 165 CAS; (s) H. Ishibashi, K. Ishihara and H. Yamamoto, J. Am. Chem. Soc., 2004, 126, 11122 CrossRef CAS PubMed; (t) K. Liu, A. Chougnet and W. D. Woggon, Angew. Chem., Int. Ed., 2008, 47, 5827 CrossRef CAS PubMed; (u) Y. K. Chung and G. C. Fu, Angew. Chem., Int. Ed., 2009, 48, 2225 CrossRef CAS PubMed.
- I. B. Masesane and Z. Y. Desta, Beilstein J. Org. Chem., 2012, 8, 2166 CrossRef CAS PubMed.
- (a) N. R. Mente, J. D. Neighbors and D. F. Wiemer, J. Org. Chem., 2008, 73, 7963 CrossRef CAS PubMed; (b) J. Chapelat, A. Buss, A. Chougnet and W. D. Woggon, Org. Lett., 2008, 10, 5123 CrossRef CAS PubMed; (c) R. Marcos, C. Rodríguez-Escrich, C. I. Herrerías and M. I. Pericas, J. Am. Chem. Soc., 2008, 130, 16838 CrossRef CAS PubMed; (d) R. S. Khupse and P. W. Erhardt, Org. Lett., 2008, 10, 5007 CrossRef CAS PubMed.
- (a) L. F. Tietze, K. M. Sommer, J. Zinngrebe and F. Stecker, Angew. Chem., Int. Ed., 2004, 43, 262 Search PubMed; (b) L. F. Tietze, F. Stecker, J. Zinngrebe and K. M. Sommer, Chem.–Eur. J., 2006, 12, 8770 CrossRef CAS PubMed.
- (a) B. M. Trost and D. F. Toste, J. Am. Chem. Soc., 1998, 120, 9074 CrossRef CAS; (b) B. M. Trost, H. C. Shen and J. P. Surviet, Angew. Chem., Int. Ed., 2003, 42, 3943 CrossRef CAS PubMed; (c) B. M. Trost, H. C. Shen and J. P. Surivet, J. Am. Chem. Soc., 2003, 125, 9276 CrossRef CAS PubMed.
- K. Fukamizu, Y. Miyake and Y. Nishibayashi, J. Am. Chem. Soc., 2008, 130, 10498 CrossRef CAS PubMed.
- R. R. R. Taylor and R. A. Batey, J. Org. Chem., 2013, 78, 1404 CrossRef CAS PubMed.
- Y. Yamamoto and K. Itonaga, Org. Lett., 2009, 11, 717 CrossRef CAS PubMed.
- J. Barluenga, M. Trincado, E. Rubio and J. M. González, J. Am. Chem. Soc., 2004, 126, 3416 CrossRef CAS PubMed.
- (a) Y. L. Shi and M. Shi, Org. Biomol. Chem., 2007, 5, 1499 RSC; (b) G. X. Wang, N. X. Wang, S. Tang, J. L. Yu and X. L. Tang, Progr. Chem., 2008, 20, 518 CAS; (c) H. C. Shen, Tetrahedron, 2009, 65, 3931 CrossRef CAS.
- Q.-L. Hua, C. Li, X.-F. Wang, L.-Q. Lu, J. R. Chen and W.-J. Xiao, ACS Catal., 2011, 1, 221 CrossRef CAS.
- X.-F. Wang, J. An, X.-X. Zhang, F. Tan, J.-R. Chen and W.-J. Xiao, Org. Lett., 2011, 13, 808 CrossRef CAS PubMed.
- W. Yang, Y. Yang and D.-M. Du, Org. Lett., 2013, 15, 1190 CrossRef CAS PubMed.
- L. Zu, S. Zhang, H. Xie and W. Wang, Org. Lett., 2009, 11, 1627 CrossRef CAS PubMed.
- V. Yu. Korotaev, I. B. Kutyashev and V. Y. Sosnovskikh, Heteroat. Chem., 2005, 16, 492 CrossRef CAS.
- (a) V. Y. Korotaev, V. Y. Sosnovskikh, I. B. Kutyashev and M. I. Kodess, Lett. Org. Chem., 2005, 2, 616 CrossRef CAS; (b) V. Y. Korotaev, I. B. Kutyashev, V. Y. Sosnovskikh and M. I. Kodess, Mendeleev Commun., 2007, 17, 52 CrossRef CAS.
- D.-Q. Xu, Y.-F. Wang, S. P. Luo, S. Zhang, A.-G. Zhong, H. Chen and Z.-Y. Xua, Adv. Synth. Catal., 2008, 350, 2610 CrossRef CAS.
- Z. Zhang, G. Jakab and P. R. Schreiner, Synthesis, 2011, 1262 Search PubMed.
- W. Hou, B. Zheng, J. Chen and Y. Peng, Org. Lett., 2012, 14, 2378 CrossRef CAS PubMed.
- V. Y. Korotaev, V. Y. Sosnovskikh, I. B. Kutyashev, A. Y. Barkov, E. G. Matochkina and M. I. Kodess, Tetrahedron, 2008, 64, 5055 CrossRef CAS.
- M. Rueping, J. Dufour and M. S. Maji, Chem. Commun., 2012, 48, 3406 RSC.
- D. Enders, G. Urbanietz, R. Hahn and G. Raabe, Synthesis, 2012, 773 CrossRef CAS.
- D. Enders, G. Urbanietz and G. Raabe, Synthesis, 2011, 1905 CrossRef CAS.
- V. Y. Korotaev, A. Y. Barkov and V. Y. Sosnovskikh, Russ. Chem. Bull., Int. Ed., 2012, 61, 674 CrossRef CAS.
- H. S. P. Rao and K. Geetha, Tetrahedron Lett., 2009, 50, 3836 CrossRef CAS.
- K. Hu, Y. Wang, Z. Zhou and C. Tang, Tetrahedron, 2014, 70, 181 CrossRef CAS.
- Y. Gao, W. Yang and D.-M. Du, Tetrahedron: Asymmetry, 2012, 23, 339 CrossRef CAS.
- K. Hu, A. Lu, Y. Wang, Z. Zhou and C. Tang, Tetrahedron: Asymmetry, 2013, 24, 953 CrossRef CAS.
- L. Wu, Y. Wang, H. Song, L. Tang, Z. Zhou and C. Tang, Adv. Synth. Catal., 2013, 1053 CrossRef CAS.
- S. Singh, A. Srivastava and S. Samanta, Tetrahedron Lett., 2012, 53, 6087 CrossRef CAS.
- (a) L. A. van Vliet, N. Rodenhuis, D. Dijkstra, H. Wikstrom, T. A. Pugsley, K. A. Serpa, L. T. Meltzer, T. G. Heffner, L. D. Wise, M. E. Lajiness, R. M. Huff, K. Svensson, S. Sundell and M. Lundmark, J. Med. Chem., 2000, 43, 2871 CrossRef CAS PubMed; (b) B. K. Trivedi, C. J. Blankley, J. A. Bristiol, H. W. Hamilton, W. C. Patt, W. J. Kramer, S. A. Johnson, R. F. Bruns, D. M. Cohen and M. J. Ryan, J. Med. Chem., 1991, 34, 1043 CrossRef CAS PubMed; (c) A. Kumar, R. V. Salunkhe, R. A. Rane and S. Y. Dike, J. Chem. Soc., Chem. Commun., 1991, 485 RSC; (d) J. H. Clark, Chem. Rev., 1980, 80, 429 CrossRef CAS; (e) E. Fujita and Y. Nagao, Bioorg. Chem., 1977, 6, 287 CrossRef CAS; (f) T. Shono, Y. Matsumura, S. Kashimura and K. Hatanaka, J. Am. Chem. Soc., 1979, 101, 4752 CrossRef CAS.
- (a) A. Beliaev, D. A. Learmonth and P. Soares-da-Silva, J. Med. Chem., 2006, 49, 1191 CrossRef CAS PubMed; (b) L. G. Larsson, R. Noreen, L. A. Renyi, S. B. Ross, D. D. Sohn, B. E. Svensson and S. O. Thorberg, PCT Int. Appl., WO 9109853 A1 19910711, 1991.
- B. Trivedi, Eur. Pat. Appl., EP 181109 A2 19860514, 1986.
- Y. Chen, Q. Zhang, B. Zhang, P. Xia, Y. Xia, Z. Y. Yang, N. Kilgore, C. Wild, S. L. Morris-Natschka and K. H. Lee, Bioorg. Med. Chem., 2004, 12, 6383 CrossRef CAS PubMed.
- M. E. Castro, P. J. Harrison, A. Pazos and T. Sharp, J. Neurochem., 2000, 75, 755 CrossRef CAS PubMed; F. Lejuene, J.-M. Rivet, A. Gobert, H. Canton and M. J. Millan, Eur. J. Pharmacol., 1993, 240, 307 CrossRef.
- (a) B. C. Ranu and S. S. Dey, Tetrahedron, 2004, 60, 4183 CrossRef CAS; (b) N. Srivastava and B. K. Banik, J. Org. Chem., 2003, 68, 2109 CrossRef CAS PubMed; (c) M. Zahouily, Y. Abrouki and A. Rayadh, Tetrahedron Lett., 2002, 43, 7729 CrossRef CAS; (d) Y. Abrouki, M. Zahouily, A. Rayadh, B. Bahlaouan and S. Sebti, Tetrahedron Lett., 2002, 43, 8951 CrossRef CAS; (e) J. S. Yadav, B. V. S. Reddy and G. Baishya, J. Org. Chem., 2003, 68, 7098 CrossRef CAS PubMed; (f) P. Kumar, R. K. Pandey and V. R. Hegde, Synlett, 1999, 1921 CrossRef CAS; (g) S. K. Garg, R. Kumar and A. K. Chakraborti, Synlett, 2005, 1370 CAS.
- (a) C. Y. Meyers, C. Rinaldi and L. Bonoli, J. Org. Chem., 1963, 28, 2440 CrossRef CAS; (b) B. D. Tilak, H. S. Desai, C. V. Deshpande, S. K. Jain and V. M. Vaidya, Tetrahedron, 1966, 22, 7 CrossRef; (c) H. Kwart and T. J. George, J. Chem. Soc., Chem. Commun., 1970, 433 RSC; (d) W. D. Cotterill, C. J. France, R. Livingstone, J. R. Atkinson and J. Cottam, J. Chem. Soc., Perkin Trans. 1, 1972, 1, 787 RSC; (e) B. Gopalan, K. Rajagopalan, S. Swaminathan and K. K. Balasubramanian, Synthesis, 1976, 409 CrossRef CAS; (f) H. Ishibashi, H. Nakatani, H. Sakashita and M. Ikeda, J. Chem. Soc., Chem. Commun., 1987, 338 RSC; (g) Y. Ishino, M. Nakamura, I. Nishiguchi and T. Hirashima, Synlett, 1991, 633 CrossRef CAS; (h) Y. Ishino, M. Mihara and H. Kawai, Synlett, 2001, 1317 CrossRef CAS.
- (a) C. V. Ursini, G. H. M. Dias and J. A. R. Rodrigues, J. Organomet. Chem., 2005, 690, 3176 CrossRef CAS; (b) D. A. Evans, F. E. Michael, J. S. Tedrow and K. R. Campos, J. Am. Chem. Soc., 2003, 125, 3534 CrossRef CAS PubMed; (c) X. Zhang, T. Taketomi, T. Yoshizumi, H. Kumobayashi, S. Akutagawa, K. Mashima and H. Takaya, J. Am. Chem. Soc., 1993, 115, 3318 CrossRef CAS; (d) H. Honig, N. Shi and G. Polanz, Biocatalysis, 1994, 9, 61 CrossRef CAS; (e) J.-W. Xie, W. Chen, R. Li, M. Zheng, W. Du, L. Yue, Y. C. Chen, Y. Wu, J. Zhu and J. G. Deng, Angew. Chem., Int. Ed., 2007, 46, 389 CrossRef CAS PubMed; (f) D. Xue, Y. C. Chen, Q. W. Wang, L. F. Cun, J. Zhu and J. G. Deng, Org. Lett., 2005, 7, 5293 CrossRef CAS PubMed.
- R. Dodda, J. J. Goldman, T. Mandal, C.-G. Zhao, G. A. Broker and E. R. T. Tiekinka, Adv. Synth. Catal., 2008, 350, 537 CrossRef CAS PubMed.
- J. Wang, H. Xie, H. Li, L. Zu and W. Wang, Angew. Chem., Int. Ed., 2008, 47, 4177 CrossRef CAS PubMed.
- (a) F. Pfrengle and H.-U. Reissig, Chem. Soc. Rev., 2010, 39, 549 RSC; (b) T. L. Gilchrist, Chem. Soc. Rev., 1983, 12, 53 RSC; (c) I. M. Lyapkalo and S. L. Ioffe, Russ. Chem. Rev., 1998, 67, 467 CrossRef; (d) P. G. Tsoungas, Heterocycles, 2002, 57, 915 CrossRef CAS; (e) P. G. Tsoungas, Heterocycles, 2002, 57, 1149 CrossRef CAS; (f) J. Santos, R. Ignacio, D. Aparicio, F. Palacios and J. M. Ezpeleta, J. Org. Chem., 2009, 74, 3444 CrossRef PubMed; (g) A. A. Tabolin, A. V. Lesiv, Y. A. Khomutova, Y. V. Nelyubina and S. L. Ioffe, Tetrahedron, 2009, 65, 4578 CrossRef CAS.
- H. Terano, S. Takase, J. Hosoda and M. Kohsaka, J. Antibiot., 1989, 42, 145 CrossRef CAS PubMed; Y. Lin, Z. Shao, G. Jiang, S. Zhou, J. Cai, L. P. Vrijmoed and E. B. Jones, Tetrahedron, 2000, 56, 9607 CrossRef.
- V. J. Lee and R. B. Woodward, J. Org. Chem., 1979, 44, 2487 CrossRef CAS.
- I. Uchida, S. Takase, H. Kayakiri, S. Kiyoto, M. Hashimoto, T. Tada, S. Koda and Y. Morimoto, J. Am. Chem. Soc., 1987, 109, 4108 CrossRef CAS; M. Iwami, S. Kiyoto, H. Terano, M. Kohsaka, H. Aoki and H. Imanaka, J. Antibiot., 1987, 40, 589 CrossRef PubMed; S. Kiyoto, T. Shibata, M. Yamashita, T. Komori, M. Okuhara, H. Terano, M. Kohsaka, H. Aoki and H. Imanaka, J. Antibiot., 1987, 40, 594 CrossRef PubMed; K. Shimomura, O. Hirai, T. Mizota, S. Matsumoto, J. Mori, F. Shibayama and H. Kikuchi, J. Antibiot., 1987, 40, 600 CrossRef PubMed.
- (a) B. Bressel, B. Egart, A. Al-Harrasi, R. Pulz, H. U. Reissig and I. Brüdgam, Eur. J. Org. Chem., 2008, 467 CrossRef CAS; (b) M. Jasiński, D. Lentz and H. U. Reissig, Beilstein J. Org. Chem., 2012, 8, 662 CrossRef PubMed; (c) B. Bressel and H. U. Reissig, Org. Lett., 2009, 11, 527 CrossRef CAS PubMed.
- (a) I. S. Young and M. A. Kerr, Org. Lett., 2004, 6, 139 CrossRef CAS PubMed; (b) M. D. Ganton and M. A. Kerr, J. Org. Chem., 2004, 69, 8554 CrossRef CAS PubMed; (c) D. A. Dias and M. A. Kerr, Org. Lett., 2009, 11, 3694 CrossRef CAS PubMed; (d) I. S. Young and M. A. Kerr, Angew. Chem., Int. Ed., 2003, 26, 3023 CrossRef PubMed.
- (a) D. Zhu, M. Lu, P. J. Chua, B. Tan, F. Wang, X. Yang and G. Zhong, Org. Lett., 2008, 10, 4585 CrossRef CAS PubMed; (b) S. Kumarn, D. M. Shaw, D. A. Longbottom and S. V. Ley, Org. Lett., 2005, 7, 4189 CrossRef CAS PubMed; (c) H. Lin, Y. Tan, X. W. Sun and G.-Q. Lin, Org. Lett., 2012, 14, 3818 CrossRef CAS PubMed.
- M. Lu, D. Zhu, Y. Lu, Y. Hou, B. Tan and G. Zhong, Angew. Chem., Int. Ed., 2008, 47, 10187 CrossRef CAS PubMed.
- (a) S. E. Denmark and A. Thorarensen, Chem. Rev., 1996, 96, 137 CrossRef CAS PubMed and the reference are cited therein; (b) S. E. Denmark and L. Gomez, J. Org. Chem., 2003, 68, 8015 CrossRef CAS PubMed; (c) A. A. Tishkov, A. V. Lesiv, Y. A. Khomutova, Y. A. Strelenko, I. D. Nesterov, M. Y. Antipin, S. L. Ioffe and S. E. Denmark, J. Org. Chem., 2003, 68, 9477 CrossRef CAS PubMed; (d) S. E. Denmark and M. Seierstad, J. Org. Chem., 1999, 64, 1610 CrossRef CAS PubMed.
- V. Y. Korotaev, V. Y. Sosnovskikh, M. A. Barabanov, E. S. Yasnova, M. A. Ezhikova, M. I. Kodess and P. A. Slepukhin, Tetrahedron, 2010, 66, 1404 CrossRef CAS.
- E. Wada and M. Yoshinaga, Tetrahedron Lett., 2004, 45, 2197 CrossRef CAS.
- F. Fringuelli, M. Matteucci, O. Piermatti, F. Pizzo and M. C. Burla, J. Org. Chem., 2001, 66, 4661 CrossRef CAS PubMed.
- R. Ballini, G. Bosica, S. Gabrielli and A. Palmieri, Tetrahedron, 2009, 65, 2916 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |