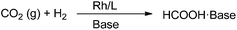An integrated process of CO2 capture and in situ hydrogenation to formate using a tunable ethoxyl-functionalized amidine and Rh/bisphosphine system†
Yu-Nong Li,
Liang-Nian He*,
Xian-Dong Lang,
Xiao-Fang Liu and
Shuai Zhang
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin, 300071, People's Republic of China. E-mail: heln@nankai.edu.cn; Fax: +86-22-2350-3878; Tel: +86-22-2350-3878
First published on 17th September 2014
Abstract
An integrated process of CO2 capture and in situ hydrogenation into formate was achieved in 95–99% yield using a tunable ethoxyl-functionalized amidine and Rh/bisphosphine system, being regarded as an alternative carbon capture and utilization approach to supply fuel-related products, to circumvent the energy penalty in carbon capture and storage. CO2 was captured by non-volatile amidine derivatives with simultaneous activation to form zwitterionic amidinium carbonate, and subsequent hydrogenation was facilitated by Rh/bisphosphine. The adsorption capacity and hydrogenation efficiency can be optimized by tuning the ethoxyl side chain. Particularly, the alkanolamidine bearing an intramolecular hydrogen donor derived from 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) gave both a high CO2 uptake (molar ratio of 0.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and excellent hydrogenation yield (99%). Furthermore, the silica-supported alkanolamidine was readily recovered and reused with the retention of good performance. This kind of carbon capture and utilization pathway could be a potential energy-saving option for industrial upgrading of CO2 from waste to fuel-related products in a carbon neutral manner.
1) and excellent hydrogenation yield (99%). Furthermore, the silica-supported alkanolamidine was readily recovered and reused with the retention of good performance. This kind of carbon capture and utilization pathway could be a potential energy-saving option for industrial upgrading of CO2 from waste to fuel-related products in a carbon neutral manner.
Introduction
Among numerous schemes to reduce greenhouse gas emissions, carbon capture and storage (CCS) is regarded as one of the most potential measures.1 However, extensive energy consumption in the desorption/compression process would be a crucial barrier for practical CCS.1c Consequently, carbon capture and utilization (CCU) has been proposed as an alternative approach to circumvent the energy issues in CCS, whereby the captured CO2 can be used as a non-toxic, abundant, and sustainable feedstock to produce value-added compounds/fuels/materials.2 In this context, a high CCU efficiency would offer feasible applications in industry. An elegant combination of CO2 capture and in situ hydrogenation to fuel-related products including formic acid, methanol, and amides, as well as methylated compounds,3 is an appealing strategy to supply renewable energy in a carbon neutral cycle.4 In addition, this protocol can avoid high-pressure operations, which are commonly required for hydrogenation reactions involving CO2. Formic acid is an extremely attractive liquid hydrogen source and hydrogen carrier for easy storage and transportation.5 An organic or inorganic base6 is always indispensable to make CO2 hydrogenation thermodynamically favorable by forming the acid–base adduct.7 Furthermore, nonvolatile amino-functional ionic liquids (ILs) and an immobilized base can be applied for this purpose to improve base recovery.8 Recently, Hicks et al. employed a double-base system consisting of polyethyleneimine (PEI)/Et3N for CO2 capture and hydrogenation, respectively. But in this system, the additional base Et3N was necessary for the hydrogenation.9 Base strength and solubility limitations can affect the hydrogenation outcome.10 In this aspect, amidine compounds have viable basicity and CO2-affinity, despite few-documented instances.10,11 Moreover, based on Jessop's switchable solvent theory,12 amidine compounds can also be used for CO2 capture in conjunction with a proton donor to form amidinium carbonate.13 In our previous work, in situ hydrogenation of the captured CO2 by PEI and a Rh/monophosphine system was achieved in yield of 37%. Notably, ammonium carbonate exhibited better activity than ammonium carbamate.2c Therefore, it is desirable and assumable to improve catalytic performance for CCU by employing the amidine compound as both the CO2 absorbent and hydrogenation promoter, which would presumably go through amidinium carbonate hydrogenation.As expected, CCU efficiency is closely related to both capture capacity and hydrogenation activity. Higher adsorption enthalpy is allowed to attain a larger capacity.14 Hence, approaches associated with enhancing the basicity e.g. introducing an electron-donating substituent have been developed to increase the adsorption capacity.14,15 In this respect, amidine compounds can be designed as a base for CO2 capture due to its high basicity and facile structural modification. More importantly, compared with the published dual-component adsorption systems, amidines with an alkyl chain bearing a hydroxyl group would remove the need of an additional volatile proton donor.16 An ethoxyl or polyethylene glycol (PEG) substituent could also promote the hydrogenation by adjusting the viscosity and facilitating mass transfer because of their electron-donating, CO2-philic and thermal-stable properties.17
We have developed non-volatile amidine derivatives based on the framework of DBU and 1,1,3,3-tetramethylguanidine (TMG) with an ethoxyl-functionalized chain bearing a terminal hydroxyl or methoxy group to tune both CO2 uptake and hydrogenation activity resulting in remarkably enhanced CCU efficiency. The amidine derivatives in this study are designated as DBUPEG150Me, TMGPEG150Me, DBUOH, DBUOH@Silica, DBU@PS (PS = Polystyrene), and DBU2PEG150Br2 as shown in Scheme 1. Herein, the amidine derivatives will serve as a “hinge base” for initial CO2 capture as an amidinium carbonate species with simultaneous activation and subsequent hydrogenation. This strategy was validated to produce formate in 95–99% yield relative to the captured CO2 i.e. the amidinium carbonate, whose hydrogenation was promoted by RhCl3·3H2O/bis(2-diphenylphosphine phenyl)ether (DPEphos) under mild conditions. Especially, a CCU industrial process for CO2 capture and in situ hydrogenation along with separation and recycling could be envisioned to avoid desorption, allowing recycling of the absorbent/base as depicted in Fig. 1.
Results and discussion
CO2 capture
Amidine derivatives were initially studied for CO2 capture. In this step, protonation occurs presumably at the sp2 nitrogen of the amidine derivative, while the ethoxyl-functionalized chain offers additional stabilization for the amidinium cation, facilitating the deprotonated oxygen species to fix CO2. Remarkably, the enhancement of CO2-philicity and basicity by the tunable structure of the ethoxyl chain could promote CO2 sorption with increasing capacity.18 Generally, more ethoxyl linkages could increase the CO2-philicity and electron-donating ability, but longer ethoxyl chains may also increase the viscosity.19 Hence, DBUPEG150Me and TMGPEG150Me with three C–C–O units, successfully approached equimolar CO2-capture with a molar ratio of 0.89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1.11
1 and 1.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, owing to their appropriate basicity (entries 1 and 2, Table 1).
1, respectively, owing to their appropriate basicity (entries 1 and 2, Table 1).
| Entry | Absorbent system (mmol mmol−1) | t (min)b | Capacityc |
|---|---|---|---|
| a Amidine derivative (3 mmol), 25 °C.b Time required to reach adsorption equilibrium unless otherwise stated.c Moles of CO2 captured per mole of superbase subtracting the solvents physical adsorption.d Using DBUOH (3 mmol) without solvent.e DBUOH@PS (0.18 g, containing 0.24 mmol DBU) Elemental analysis: N 3.70%, DBU 20.0% in weight.f DBUOH@Silica (0.42 g, containing 0.24 mmol DBUOH). Elemental analysis: N 1.60%, DBU 8.69% or DBUOH 11.3% in weight. | |||
| 1 | DBUPEG150Me/glycol (3/6) | 12 | 0.89 |
| 2 | TMGPEG150Me/glycol (3/18) | 9 | 1.11 |
| 3 | DBU2PEG150Br2/glycol (3/24) | 27 | — |
| 4d | DBUOH (3) | 9 | 0.58 |
| 5 | DBUOH/TGDE (3/5) | 27 | 0.95 |
| 6e | DBU@PS/glycol (0.24/24) | 18 | 0.90 |
| 7f | DBUOH@ Silica/TGDE (0.24/5) | 9 | 3.02 |
| 8f | DBUOH@ silica/glycol (0.24/24) | 15 | 3.48 |
In this context, the characteristic signals at 151.29 and 158.73 ppm in the 13C NMR spectra support the formation of amidinium alkylcarbonates. The bands centered at 1588, 1405 and 1294 cm−1 in the FT-IR spectra can be assigned to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of carbonate, basically in agreement with those previously reported13c (see ESI†). But DBU2PEG150Br2 exhibited no adsorption capacity due to its extremely weak basicity and high viscosity (entry 3). Furthermore, alkanolamidine (DBUOH) with one ethoxyl unit, containing an equal number of amidine and intramolecular hydroxyl groups, can almost capture an equimolar amount of CO2 in the absence of an additional proton donor (entry 4 and 5). Here, tetraethylene glycol dimethyl ether (TGDE) was used to adjust the viscosity of DBUOH. In the 13C NMR spectrum, the additional peak at 164.25 ppm likely corresponds to the intramolecular amidinium carbonate. Besides, the strong band of [C
O bond of carbonate, basically in agreement with those previously reported13c (see ESI†). But DBU2PEG150Br2 exhibited no adsorption capacity due to its extremely weak basicity and high viscosity (entry 3). Furthermore, alkanolamidine (DBUOH) with one ethoxyl unit, containing an equal number of amidine and intramolecular hydroxyl groups, can almost capture an equimolar amount of CO2 in the absence of an additional proton donor (entry 4 and 5). Here, tetraethylene glycol dimethyl ether (TGDE) was used to adjust the viscosity of DBUOH. In the 13C NMR spectrum, the additional peak at 164.25 ppm likely corresponds to the intramolecular amidinium carbonate. Besides, the strong band of [C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH]+ at 1646 cm−1, comparable to that of [HDBU]Cl (1645 cm−1),12,20 and the peaks corresponding to C
NH]+ at 1646 cm−1, comparable to that of [HDBU]Cl (1645 cm−1),12,20 and the peaks corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1554, 1421 and 1297 cm−1 can also be observed (see ESI†).
O at 1554, 1421 and 1297 cm−1 can also be observed (see ESI†).
DFT calculated enthalpy changes revealed that the intramolecular carbonate as the adsorption product may undergo a cyclic formation via hydrogen bonding (Fig. S1, ESI†). Furthermore, the polystyrene supported DBU/glycol system was able to absorb CO2 in a molar ratio of 0.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 6). Particularly, in the aprotic solvent, TGDE, 0.42 g of DBUOH@Silica (Fig. S4 and S6, ESI†) can absorb 0.73 mmol of CO2, corresponding to a capacity of 76 mg g−1 gravimetrically. The maximum adsorption ratio can be 3.48
1 (entry 6). Particularly, in the aprotic solvent, TGDE, 0.42 g of DBUOH@Silica (Fig. S4 and S6, ESI†) can absorb 0.73 mmol of CO2, corresponding to a capacity of 76 mg g−1 gravimetrically. The maximum adsorption ratio can be 3.48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (88 mg g−1) using DBUOH@Silica in glycol (entries 7 and 8). In the chemisorption process, the maximum adsorption ratio should be around 1.
1 (88 mg g−1) using DBUOH@Silica in glycol (entries 7 and 8). In the chemisorption process, the maximum adsorption ratio should be around 1.
In addition, DBUOH@silica as a solid absorbent can physically absorb CO2 besides chemisorption. Thus, the actual adsorption ratio reached more than 1 relative to the effective amount of the 0.24 mmol superbase DBUOH@silica contains. Similarly, the peaks at 1646, 1538, 1456, and 1292 cm−1, and a decrease in the OH signal in the FT-IR spectrum suggest the generation of the amidinium carbonate via chemisorption (Fig. 2). In the physical adsorption process, DBUOH@Silica could successfully be utilized for adsorption and desorption measurements (Fig. 3). DBUOH@Silica can physically absorb CO2 to 6.2 cm3 g−1 (14.2 mg g−1) at 25 °C under 0.12 MPa, which undergoes desorption using a N2 flow of 0.1 L min−1 (Fig. 3a). Notably, no significant drop in CO2 adsorption was observed after three successive adsorption–desorption cycles (Fig. 3b), indicating DBUOH@Silica is stable enough to be recycled.
 | ||
| Fig. 3 Reversible CO2 adsorption and desorption (a) and three consecutive cycles (b) using DBUOH@Silica. | ||
The hydrogenation of gaseous CO2 to formate
Subsequently, gaseous CO2 hydrogenation was performed to establish an optimal catalytic system for the in situ hydrogenation of the captured CO2, which is also an activated form of CO2.The base effect on the hydrogenation of gaseous CO2
Considering the promotive effect of bases, amidines were initially compared with a range of organic and inorganic bases for gaseous CO2 hydrogenation using RhCl3/PPh3 at 100 °C. As expected, DBU, TMG, DBN (1,5-diazobicyclo[4.3.0]non-5-ene) and TBD (1,5,7-triazabicyclo[4,4,0]dec-5-ene) showed better activity than conventional amines including TEA (triethanolamine), HMTA (hexamethylenetetramine), Et3N, Et2NH, TMEDA (N,N,N′,N′-tetramethylethylenediamine), DABCO (triethylenediamine), morpholine and DMAP (4-dimethylaminopyridine). Among the amidines used in this study, DBU and TMG displayed superiority with TONs of 362 and 322, respectively (Fig. 4). Although NaOH is a strong base, only a small amount of formate was obtained owing to its solubility limitations and incompatibility with the catalytic system. Moreover, weak bases such as 1-methylimidazole, choline chloride, mono- and di-ethanolamine gave inferior results.Rh/bisphosphine-catalyzed hydrogenation of gaseous CO2
Screening the phosphine and nitrogen ligands in the catalytic system was further studied as listed in Table 2. CO2 hydrogenation almost did not occur in the absence of a phosphine ligand (entry 1, Table 2). Obviously, PPh3 exhibited better activity than PnBu3 and (2-furyl)3P (entries 2–4). Among the monophosphines derived from PPh3, the activity increases in the sequence (p-tolyl)PPh2 < PPh3 < (o-tolyl)PPh2 < Cy2PPh ≤ CyPPh2 (entries 2, 5–8), being more or less consistent with nucleophilicity. In contrast, tBu-XPhos was decisively inferior presumably due to steric hindrance (entry 9). Moreover, bisphosphines were particularly tested. 1,3-Bis(diphenyl phosphino)propane (dppp) with a flexible chain was proven to be inactive (entry 10). However, when the dppbz, BINAP, dppf and DPEphos ligands were used, the TONs were remarkably improved from 69 to 516, respectively (entries 11–14). This superiority is probably due to the electron-donating ability of their rigid chains compared to dppp, and may also be related to their cone angles when coordinating with the Rh center.21 In this regard, DPEphos has supreme merit in view of its large cone angle and strong electron-donating ability that account for the highest activity (entry 14). Though Xantphos' framework is conspicuously similar to DPEphos, it gave a lower formate yield, presumably resulting from unfavorable effects arising from a chelating coordination between its oxygen atom and the metal center (entry 14 vs. 15). Multi-phosphine, P–N ligands, and nitrogen ligands exhibited worse activity in comparison with those bisphosphines (entries 16–21). Hence, ligands with strong electron-donating ability or rigid chains, as well as an appropriate cone angle are favorable for the hydrogenation reaction.| Entry | Ligand | TONb |
|---|---|---|
| a Reaction conditions: RhCl3·3H2O (0.01 mmol, 2.6 mg), monodentate ligand (0.1 mmol) or bidentate/multidentate ligand (0.05 mmol), DBU (3.3 mmol), methanol (3 mL), H2 (4 MPa), CO2 (4 MPa), 60 °C, 16 h.b Refers to formate including the HCOO−BH+ adduct and a little HCOOMe calculated by 1H NMR spectroscopy using 1,1,2,2-tetrachloromethane as an internal standard. TON = turnover number: moles of formate (HCOO−BH+ and HCOOMe) per mole of Rh catalyst. | ||
| 1 | — | 5 |
| 2 | PPh3 | 362 |
| 3 | PnBu3 | 290 |
| 4 | P(2-furyl)3 | 337 |
| 5 | (o-Tolyl)PPh2 | 441 |
| 6 | (p-Tolyl)PPh2 | 262 |
| 7 | CyPPh2 | 480 |
| 8 | Cy2PPh | 473 |
| 9 |  |
67 |
| 10 |  |
17 |
| 11 | 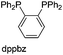 |
69 |
| 12 |  |
470 |
| 13 |  |
499 |
| 14 |  |
516 |
| 15 |  |
122 |
| 16 |  |
256 |
| 17 |  |
249 |
| 18 | 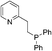 |
115 |
| 19 |  |
111 |
| 20 |  |
46 |
| 21 |  |
95 |
Moreover, Fig. S2 (see ESI†) shows that increasing the reaction temperature from 60 to 120 °C caused the product yield to drop sharply, whereas at temperatures below 40 °C it also gave inferior results (Fig. S2a†). It is probably because that increasing the temperature is detrimental to the exothermic hydrogenation of CO2, while lower temperatures may slow down the reaction. As a result, formate with a TON of 1565 can be obtained at the optimal temperature of 60 °C (Fig. S2b†). To our delight, a TON of 2202 can be successfully attained through elongating the reaction time to 32 h.
Amidine derivatives as a promoter
With the proper conditions in hand, the modified amidine derivatives were examined for RhCl3/DPEphos-catalyzed CO2 hydrogenation in order to further promote the process (Fig. 5). As is well known, the CO2-philic ethoxyl group could have impacts on the physical properties such as increased gas/liquid diffusion rates, and thus assumedly facilitates the reaction involving CO2.13c Nevertheless, viscous DBU2PEG150Br2 as an IL showed too weak basicity to stabilize formic acid, and gave a rather low yield. In order to increase the basicity, the secondary amine e.g. TMG was modified to the tertiary amine TMGPEG150Me, and DBU was also modified by an electron-donating PEG150Me substituent, which could accelerate the formation of HCOOMe. Dramatically, DBUOH as an alkanolamidine performed well with the highest TON of 1698 within 16 h, better than both DBU and DBUPEG150Me. The terminal hydroxyl group is assumed to facilitate proton transfer among the reactive species, resulting in the promotion of formate generation,22 albeit its relatively high viscosity. Notably, the silica-supported alkanolamidine (DBUOH@Silica) also exhibited a higher efficiency than the polystyrene supported derivative (DBU@PS).In situ hydrogenation of the captured CO2 (CCU)
Hydrogenation of gaseous CO2 can be efficiently achieved by the tunable ethoxyl-functionalized amidines, which stimulated us to further investigate the hydrogenation of captured CO2 by using the amidine derivatives as the “hinge base”, without the addition of extra base. The captured CO2 i.e. amidinium carbonate, could be a simultaneously activated form, which might show higher activity than the gaseous one.On the basis of the results in Table 1, hydrogenation of those amidinium carbonates formed from CO2 capture were efficiently performed as summarized in Table 3. TMGPEG150Me gave a higher formate yield in regard to captured CO2 (90%) when compared to DBUPEG150Me (65%) due to the higher basicity and lower viscosity of TMGPEG150Me (entry 1 vs. 2, Table 3). Dramatically, using DBUOH as the “hinge base”, the formate yield can be improved to 97–99% (entries 3 and 4). On the one hand, DBUOH with a terminal hydroxyl group reached equimolar CO2 capture, providing enough active CO2 source for in situ hydrogenation. On the other hand, the ethoxyl group could adjust the basicity of the DBU derivatives and the terminal OH could participate in proton transfer during the catalytic cycle. To our delight, the silica-supported DBUOH also worked effectively for in situ hydrogenation of captured CO2 to afford formate in 96–99% yield (entries 5 and 6). As a result, free formic acid can be separated by a filtration and distillation sequence. DBUOH@Silica could be recovered without changing its functionality (Fig. S3, ESI†) and was reused for at least three-successive cycles, still giving the desired product in 89% yield after the third run, although its capacity gradually decreased (entries 6–8, Table 3). Significantly, this is an efficient CCU process, removing the energy penalty step, to produce energy-related products from CO2.
| Entry | Substrate | Adsorption ratiod (mol mol−1) | Captured CO2 (mmol) | HCOO− TONb | CCU Yieldc (%) |
|---|---|---|---|---|---|
| a Reaction conditions: RhCl3·3H2O (0.01 mmol, 1.3 mg), DPEphos (0.05 mmol, 13.5 mg), methanol (2 mL), H2 (4 MPa), 60 °C, 16 h.b Refers to HCOO−BH+, determined by 1H NMR spectroscopy.c Relative to the amount of captured CO2.d Moles of CO2 captured per mole of superbase subtracting solvent physical adsorption. Adsorption ratio (g g−1) is given in brackets.e DBUPEG150Me (3 mmol)/glycol (6 mmol).f TMGPEG150Me (3 mmol)/glycol (18 mmol).g DBUOH (3 mmol).h DBUOH (3 mmol)/TGDE (5 mmol).i DBUOH@Silica (0.42 g, 0.24 mmol DBUOH)/glycol (24 mmol).j DBUOH@Silica (0.42 g, 0.24 mmol)/TGDE (5 mmol).k Run 2, DBUOH@Silica (0.42 g)/TGDE (5 mmol).l Run 3, DBUOH@Silica (0.40 g, 0.229 mmol DBUOH)/TGDE (5 mmol). | |||||
| 1e |  |
0.89 (0.132) | 2.77 | 180 | 65 |
| 2f | 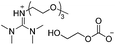 |
1.11 (0.187) | 3.59 | 323 | 90 |
| 3g |  |
0.58 (0.122) | 1.74 | 169 | 97 |
| 4h |  |
0.95 (0.199) | 3.02 | 298 | 99 |
| 5i |  |
3.48 (0.088) | 1.17 | 113 | 96 |
| 6j |  |
3.02 (0.076) | 0.92 | 91 | 99 |
| 7k | DBUOH@Silica-CO2 | 2.85 (0.072) | 0.88 | 71 | 81 |
| 8l | DBUOH@Silica-CO2 | 2.37 (0.060) | 0.74 | 66 | 89 |
Spectroscopy investigation
Deeper insight into an integration process combining CO2 capture with in situ hydrogenation using a DBUOH/RhCl3/DPEphos system was investigated by in situ FT-IR spectroscopy under CO2 pressure. In the FT-IR spectrum, the characteristic peak for CO223 at 2340 cm−1 and the appearance of carbonyl peaks at 1271, 1438 and 1577 cm−1 supports the formation of amidinium carbonate upon uptake of CO2 (Fig. 6), basically consistent with the FT-IR data mentioned above.13c A characteristic peak centered at 1647 cm−1 corresponds to the [C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH]+ band adsorption12,20 of protonated DBU. Bands at 1985 and 2006 cm−1, were assigned to the stretching vibration of intermediates with two kinds of Rh–H bonds,24 which gradually increased during the hydrogenation step. The generation of the intermediate formato-Rh complex24 was also observed at 1589 and 1312 cm−1. Broad adsorptions from 1776 to 1805 cm−1 were suggestive of formic acid derivatives.24 The detection of intermediates and products by in situ FT-IR spectroscopy would lay a strong foundation for the proposed mechanism.
NH]+ band adsorption12,20 of protonated DBU. Bands at 1985 and 2006 cm−1, were assigned to the stretching vibration of intermediates with two kinds of Rh–H bonds,24 which gradually increased during the hydrogenation step. The generation of the intermediate formato-Rh complex24 was also observed at 1589 and 1312 cm−1. Broad adsorptions from 1776 to 1805 cm−1 were suggestive of formic acid derivatives.24 The detection of intermediates and products by in situ FT-IR spectroscopy would lay a strong foundation for the proposed mechanism.
In addition, the BET (Brunauer–Emmett–Teller) surface area for the solid amidine derivatives i.e. DBU@PS and DBUOH@Silica were measured to be 27.3 and 98.5 m2 g−1, respectively (Table S1†). DBUOH@Silica showed a higher surface area than DBU@PS, and SEM (scanning electron microscopy), also indicated the formation of microparticles on the surface of DBUOH@Silica (Fig. 7). All these physical characteristics could promote the interactions between the base and other species, and improve the catalytic efficiency, thus giving higher yields in hydrogenation process.
Reaction mechanism
In spite of the well-documented mechanism on the hydrogenation of gaseous CO224,25 and CO2 derivatives,26 a tentative pathway that rationalizes the present process via initial CO2 capture and subsequent hydrogenation to formate is illustrated in Scheme 2. The Rh–H hydride 1 is initially formed from RhCl3 and DPEphos under H2 pressure. The CO2 capture product i.e. amidinium alkylcarbonate (DBUH+OCO2−) coordinates with the Rh species and thus facilitates intramolecular hydride transfer of 2 and carbonyl insertion into the Rh–H bond, generating the intermediate formato-Rh complex 3. Subsequently, 4 could be formed via the oxidative addition of H2, and eventually the reductive elimination of 5 HCOOH·DBUOH occurs, regenerating 1 to complete the catalytic cycle. In this step, DBUOH would participate in the proton transfer, which may not only promote the heterolytic cleavage and δ-bond metathesis of H2, but also provide protons to 3 in favor of reductive elimination of HCOOH. | ||
| Scheme 2 The proposed mechanism for CO2 capture and subsequent Rh-catalyzed hydrogenation of the captured CO2 using DBUOH. | ||
Experimental section
Procedure of CO2 capture
CO2 capture was carried out in a 10 mL glass tube. The absorbents were charged into the reactor at room temperature. Then, the air in the flask was replaced by CO2 and a needle used for CO2 bubbling, which was inserted in the bottom of the glass tube. The adsorption reaction was stirred and conducted with a CO2 bubbling rate of 0.1 L min−1. The amount of CO2 absorbed was determined using an analytical balance within an accuracy of ±0.0001 g every three minutes. The measurements of CO2 sorption by solid absorbent (DBUOH@Silica) were performed on ASAP 2020 Surface Area and Porosity Analyzer (a solid adsorption instrument, which can absorb CO2 and analyze the change in the amount of physical adsorption as a function of time or pressure). Before measurement, the sample was dried again using the ‘outgas’ function of the surface area analyzer for about one hour at 50 °C. Adsorption–desorption was determined by several cycles of repeated experiments.Procedure of gaseous CO2 hydrogenation
The manipulations were performed in a recirculating mBraun MBio compact inert atmosphere (Ar) drybox at 20 °C. The reaction was carried out in a 50 mL stainless-steel autoclave reactor with an inner glass tube and a magnetic stirrer. Initially, the reaction mixture, consisting of methanol, RhCl3·3H2O and the ligand, was transferred to the inner glass and stirred for 10 min. After that, the base was added dropwise to the mixture with stirring. After sealing the autoclave reactor cover, H2 and CO2 were introduced to the system with an initial pressure of 4 MPa and 4 MPa, respectively. The reactive mixture was stirred for 16–32 h at 60 °C. After the hydrogenation was complete, the autoclave was cooled to room temperature, and the excess gas depressurized very slowly in ice-water. The resultant mixture was analyzed using NMR spectroscopy with 1,1,2,2-tetrachloromethane as an internal standard. The free formic acid was separated by distillation at 160 °C.Integrated procedure of CO2 capture and in situ hydrogenation
CO2 capture was carried out in a 10 mL glass tube. The absorbents were charged into the reactor at room temperature. Then, the air in the flask was replaced by CO2 and a needle used for CO2 bubbling, which was inserted in the bottom of the glass tube. The adsorption reaction was stirred and conducted with a CO2 bubbling rate of 0.1 L min−1. The amount of CO2 absorbed was determined using an analytical balance within an accuracy of ±0.0001 g every three minutes. Subsequently, the manipulations were performed under an Ar atmosphere. The CO2 capture product was dissolved in methanol, then RhCl3·3H2O and the ligand were mixed and transferred to the glass tube, which was placed into the 50 mL stainless-steel autoclave. After sealing the autoclave reactor cover, H2 was led into the system with an initial pressure of 4 MPa. The reactive mixture was stirred for 16 h at 60 °C. The same post-processing and analysis method was used as described above.Procedure for recycling DBUOH@Silica
After the process of CO2 capture and in situ hydrogenation was completed, the solid was filtrated and washed with methanol, and the formic acid was distilled. The recovered base was a grey powder, which was further dried using a rotary evaporator and at 60 °C under vacuum. The recovered base was directly used for the next run. The reactions were performed under identical reaction conditions described above.Conclusions
In summary, the efficiency for in situ hydrogenation of captured CO2 has been improved up to more than 99% by employing an ethoxyl-functionalized amidine/Rh/DPEphos sequential system. It is worth mentioning that the ethoxyl linkages could have an electron-donating and CO2-philic effect that improves the basicity and adjusts the viscosity of the amidine, while the terminal OH group of DBUOH could also lead to desirable proton transfer. Therefore, introducing ethoxyl chains could promote both capture efficiency and hydrogenation activity. This protocol offers a non-volatile, quantitative adsorption with simultaneous activation to form zwitterionic amidinium carbonate, and then in situ Rh-bisphosphine-catalyzed hydrogenation of the captured CO2 can be performed smoothly. Particularly, the recyclable silica-supported alkanolamidine gave high activity without using a volatile proton donor, thereby validating the CCU strategy successfully, which was investigated by NMR and in situ FT-IR spectroscopy, as well as TGA, BET, SEM and DFT techniques. This efficient CCU concept could also have the potential practice in industry, circumventing the energy input for the desorption step and generating energy-related products.Acknowledgements
This work was financially supported by the National Natural Sciences Foundation of China, Specialized Research Fund for the Doctoral Program of Higher Education (20130031110013), and MOE Innovation Team (IRT13022) of China.References
- For CCS, see: (a) D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed; (b) P. Markewitz, W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, A. Schreiber and T. E. Muller, Energy Environ. Sci., 2012, 5, 7281–7305 RSC; (c) M. E. Boot-Handford, J. C. Abanades, E. J. Anthony, M. J. Blunt, S. Brandani, N. Mac Dowell, J. R. Fernandez, M.-C. Ferrari, R. Gross, J. P. Hallett, R. S. Haszeldine, P. Heptonstall, A. Lyngfelt, Z. Makuch, E. Mangano, R. T. J. Porter, M. Pourkashanian, G. T. Rochelle, N. Shah, J. G. Yao and P. S. Fennell, Energy Environ. Sci., 2014, 7, 130–189 RSC.
- For CCU, see: (a) Z.-Z. Yang, L.-N. He, J. Gao, A.-H. Liu and B. Yu, Energy Environ. Sci., 2012, 5, 6602–6639 RSC; (b) A.-H. Liu, R. Ma, C. Song, Z.-Z. Yang, A. Yu, Y. Cai, L.-N. He, Y.-N. Zhao, B. Yu and Q.-W. Song, Angew. Chem., Int. Ed., 2012, 51, 11306–11310 CrossRef CAS PubMed; (c) Y.-N. Li, L.-N. He, A. H. Liu, X.-D. Lang, Z.-Z. Yang, B. Yu and C.-R. Luan, Green Chem., 2013, 15, 2825–2829 RSC; (d) S. H. Kim, K. H. Kim and S. H. Hong, Angew. Chem., Int. Ed., 2014, 53, 771–774 CrossRef CAS PubMed; (e) S. Zhang, Y.-N. Li, Y.-W. Zhang, L.-N. He, B. Yu, Q.-W. Song and X.-D. Lang, ChemSusChem, 2014, 7, 1484–1489 CrossRef CAS PubMed.
- For fuel-related products of CO2 hydrogenation, see: (a) Y.-N. Li, R. Ma, L.-N. He and Z.-F. Diao, Catal. Sci. Technol., 2014, 4, 1498–1512 RSC; (b) K. Beydoun, T. vom Stein, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2013, 52, 9554–9557 CrossRef CAS PubMed; (c) Y. Li, X. Fang, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 9568–9571 CrossRef CAS PubMed; (d) B. Yu, H. Zhang, Y. Zhao, S. Chen, J. Xu, C. Huang and Z. Liu, Green Chem., 2013, 15, 95–99 RSC; (e) B. Yu, Y. Zhao, H. Zhang, J. Xu, L. Hao, X. Gao and Z. Liu, Chem. Commun., 2014, 50, 2330–2333 RSC; (f) J. H. Kwak, L. Kovarik and J. Szanyi, ACS Catal., 2013, 3, 2094–2100 CrossRef CAS.
- For carbon neutral cycle, see: (a) G. A. Olah, Angew. Chem., Int. Ed., 2013, 52, 104–107 CrossRef CAS PubMed; (b) G. A. Olah, A. Goeppert and G. K. S. Prakash, J. Org. Chem., 2009, 74, 487–498 CrossRef CAS PubMed; (c) G. A. Olah, Angew. Chem., Int. Ed., 2005, 44, 2636–2639 CrossRef CAS PubMed.
- For formic acid as hydrogen carrier, see: (a) H.-L. Jiang, S. K. Singh, J.-M. Yan, X.-B. Zhang and Q. Xu, ChemSusChem, 2010, 3, 541–549 CrossRef CAS PubMed; (b) S. Enthaler, ChemSusChem, 2008, 1, 801–804 CrossRef CAS PubMed; (c) J. F. Hull, Y. Himeda, W.-H. Wang, B. Hashiguchi, R. Periana, D. J. Szalda, J. T. Muckerman and E. Fujita, Nat. Chem., 2012, 4, 383–388 CrossRef CAS PubMed.
- For base-promoted CO2 hydrogenation, see: (a) R. Tanaka, M. Yamashita and K. Nozaki, J. Am. Chem. Soc., 2009, 131, 14168–14169 CrossRef CAS PubMed; (b) P. G. Jessop, Y. Hsiao, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1996, 118, 344–355 CrossRef CAS; (c) C. Das Neves Gomes, O. Jacquet, C. Villiers, P. Thuéry, M. Ephritikhine and T. Cantat, Angew. Chem., Int. Ed., 2012, 51, 187–190 CrossRef CAS PubMed; (d) H. Hayashi, S. Ogo, T. Abura and S. Fukuzumi, J. Am. Chem. Soc., 2003, 125, 14266–14267 CrossRef CAS PubMed; (e) T. J. Schmeier, G. E. Dobereiner, R. H. Crabtree and N. Hazari, J. Am. Chem. Soc., 2011, 133, 9274–9277 CrossRef CAS PubMed; (f) Z. Xu, N. D. McNamara, G. T. Neumann, W. F. Schneider and J. C. Hicks, ChemCatChem, 2013, 5, 1769–1771 CrossRef CAS; (g) G. A. Filonenko, M. P. Conley, C. Copéret, M. Lutz, E. J. M. Hensen and E. A. Pidko, ACS Catal., 2013, 3, 2522–2526 CrossRef CAS.
- T. Schaub and R. A. Paciello, Angew. Chem., Int. Ed., 2011, 50, 7278–7282 CrossRef CAS PubMed.
- (a) S. Wesselbaum, U. Hintermair and W. Leitner, Angew. Chem., Int. Ed., 2012, 51, 8585–8588 CrossRef CAS PubMed; (b) Z. Zhang, Y. Xie, W. Li, S. Hu, J. Song, T. Jiang and B. Han, Angew. Chem., Int. Ed., 2008, 47, 1127–1129 CrossRef CAS PubMed; (c) Z. Zhang, S. Hu, J. Song, W. Li, G. Yang and B. Han, ChemSusChem, 2009, 2, 234–238 CrossRef CAS PubMed.
- N. D. McNamara and J. C. Hicks, ChemSusChem, 2014, 7, 1114–1124 CrossRef CAS PubMed.
- P. Munshi, A. D. Main, J. C. Linehan, C.-C. Tai and P. G. Jessop, J. Am. Chem. Soc., 2002, 124, 7963–7971 CrossRef CAS PubMed.
- (a) C.-C. Tai, T. Chang, B. Roller and P. G. Jessop, Inorg. Chem., 2003, 42, 7340–7341 CrossRef CAS PubMed; (b) R. J. Perry, T. A. Grocela-Rocha, M. J. O'Brien, S. Genovese, B. R. Wood, L. N. Lewis, H. Lam, G. Soloveichik, M. Rubinsztajn, S. Kniajanski, S. Draper, R. M. Enick, J. K. Johnson, H.-b. Xie and D. Tapriyal, ChemSusChem, 2010, 3, 919–930 CrossRef CAS PubMed.
- P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS PubMed.
- For superbase and proton donor systems, see: (a) C. Wang, H. Luo, X. Luo, H. Li and S. Dai, Green Chem., 2010, 12, 2019–2023 RSC; (b) C. Wang, H. Luo, D. Jiang, H. Li and S. Dai, Angew. Chem., 2010, 122, 6114–6117 CrossRef; (c) Z.-Z. Yang, L.-N. He, Y.-N. Zhao, B. Li and B. Yu, Energy Environ. Sci., 2011, 4, 3971–3975 RSC.
- G. Cui, J. Zheng, X. Luo, W. Lin, F. Ding, H. Li and C. Wang, Angew. Chem., 2013, 125, 10814–10818 CrossRef.
- B. Gurkan, B. F. Goodrich, E. M. Mindrup, L. E. Ficke, M. Massel, S. Seo, T. P. Senftle, H. Wu, M. F. Glaser, J. K. Shah, E. J. Maginn, J. F. Brennecke and W. F. Schneider, J. Phys. Chem. Lett., 2010, 1, 3494–3499 CrossRef CAS.
- (a) M. Kim and J.-W. Park, Chem. Commun., 2010, 46, 2507–2509 RSC; (b) P. K. Koech, J. Zhang, I. V. Kutnyakov, L. Cosimbescu, S.-J. Lee, M. E. Bowden, T. D. Smurthwaite and D. J. Heldebrant, RSC Adv., 2013, 3, 566–572 RSC.
- (a) D. J. Heldebrant and P. G. Jessop, J. Am. Chem. Soc., 2003, 125, 5600–5601 CrossRef CAS PubMed; (b) Y.-N. Li, J.-L. Wang and L.-N. He, Tetrahedron Lett., 2011, 52, 3485–3488 CrossRef CAS PubMed.
- Z.-Z. Yang, L.-N. He, Y.-N. Zhao and B. Yu, Environ. Sci. Technol., 2013, 47, 1598–1605 CAS.
- Z.-Z. Yang, Q.-W. Song and L.-N. He, Capture and utilization of carbon dioxide with polyethylene glycol, Springer, Heidelberg, New York, Dordrecht, London, 2012, p. 78 Search PubMed.
- D. J. Heldebrant, P. K. Koech, M. T. C. Ang, C. Liang, J. E. Rainbolt, C. R. Yonker and P. G. Jessop, Green Chem., 2010, 12, 713–721 RSC.
- C.-C. Tai, J. Pitts, J. C. Linehan, A. D. Main, P. Munshi and P. G. Jessop, Inorg. Chem., 2002, 41, 1606–1614 CrossRef CAS PubMed.
- (a) C. Yin, Z. Xu, S. Yang, S. Ng, K. Wong, Z. Lin and C. Lau, Organometallics, 2001, 20, 1216–1222 CrossRef CAS; (b) Y. Himeda, N. Onozawa-Komatsuzaki, H. Sugihara, H. Arakawa and K. Kasuga, Organometallics, 2004, 23, 1480–1483 CrossRef CAS.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef CAS PubMed.
- J. C. Tsai and K. M. Nicholas, J. Am. Chem. Soc., 1992, 114, 5117–5124 CrossRef CAS.
- Y. Musashi and S. Sakaki, J. Am. Chem. Soc., 2002, 124, 7588–7603 CrossRef CAS PubMed.
- (a) E. Balaraman, C. Gunanathan, J. Zhang, L. J. W. Shimon and D. Milstein, Nat. Chem., 2011, 3, 609–614 CrossRef CAS PubMed; (b) Z. Han, L. Rong, J. Wu, L. Zhang, Z. Wang and K. Ding, Angew. Chem., Int. Ed., 2012, 51, 13041–13045 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental methods, experimental procedures and results, characterization for adsorption and hydrogenation systems, and copies of NMR, in situ FTIR, FT-IR, GC-MS spectra, TGA and DFT study. See DOI: 10.1039/c4ra08740b |
| This journal is © The Royal Society of Chemistry 2014 |