The effect of K2HPO4 and Al2(SO4)3 modified MCM-41 on the dehydration of methyl lactate to acrylic acid
Bin Wang*,
Chao li,
Qiangqiang Zhu and
Tianwei Tan
Beijing Key Lab of Bioprocess, College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: wangbin@mail.buct.edu.cn
First published on 8th September 2014
Abstract
In this paper, Al2(SO4)3 and K2HPO4 modified MCM-41 were developed as catalysts for efficient conversion of methyl lactate (ML) to acrylic acid (AA) and methyl acrylate (MA). By changing the loading amount of the two salts and their ratio, the catalytic performance was optimized and the highest AA + MA yield of 73.6% was achieved over the 45 wt% K2HPO4 and Al2(SO4)3 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)/MCM-41 catalyst at 410 °C. The physicochemical properties of the catalysts were investigated by various techniques including XRD, BET, NH3-TPD, CO2-TPD, pyridine adsorption-FTIR and CO2 adsorption-FTIR. The 45% loading sample showed the smallest acidic amounts and a moderate basicity amount, and the acidic–basicity ratio was thought to be crucial for the AA selectivity. Further decrease or increase in the ratio would cause a drop in AA selectivity. Carbon deposition was the main reason for catalyst deactivation. The catalyst could be fully regenerated by air treatment and behaved with good activity after 10 regenerations.
4)/MCM-41 catalyst at 410 °C. The physicochemical properties of the catalysts were investigated by various techniques including XRD, BET, NH3-TPD, CO2-TPD, pyridine adsorption-FTIR and CO2 adsorption-FTIR. The 45% loading sample showed the smallest acidic amounts and a moderate basicity amount, and the acidic–basicity ratio was thought to be crucial for the AA selectivity. Further decrease or increase in the ratio would cause a drop in AA selectivity. Carbon deposition was the main reason for catalyst deactivation. The catalyst could be fully regenerated by air treatment and behaved with good activity after 10 regenerations.
1. Introduction
Acrylic acid is an extremely valuable chemical intermediate used to produce paint additives, adhesives, textile and leather treating agents and other products.1–4 Oxidation of propene to acrylic acid is a commercial process5 which is the major contributor for AA production. In recent years, the partial oxidation of propane to AA through a one-step process has drawn much attention.5–8 However, all of the above routes are based on fossil resources. In recent years, the reduction of CO2 emission has become a crucial problem in the chemical industry. Given the current robust forces driving sustainable chemical production and available biomass conversion technologies, biomass-based routes are expected to make a significant impact on the chemical industry for producing bulk chemicals in the future.9–11 Methyl lactate and lactic acid are commodity chemicals produced from renewable biomass resources.12–15 Dehydration of LA or ML to AA is an environmental protection rout, but there still remain some difficulties: besides the dehydration of LA or ML to achieve AA, some side reactions such as decarbonylation/decarboxylation, condensation, hydrogenation can also occur, which lead to low AA selectivity.16 Moreover, compared with the dehydration reaction, decarbonylation/decarboxylation is a thermodynamic priority reaction. Therefore, the development of an efficient catalyst for dehydration is necessary and crucial for an eco-friendly and cost-effective process.In the literature, several catalysts consisting chiefly of sulphates and phosphates of the group I and II metals, supported phosphates salts on NaY and calcium hydroxyapatites have been reported.17–24 The catalytic dehydration rate of alcohol over catalyst is thought to be dependent on the catalyst surface properties. The dehydration of lactic acid over various metal sulphates was investigated by Tang. BaSO4 was found to show an efficient activity with 99.8% lactic acid conversion and 74.0% acrylic acid selectivity.25 The high activity was ascribed to the moderate acidity on its surface. This view was also supported by Huang.26 The effect of rare earth metals (lanthanum, cerium, samarium, and europium) supported on NaY catalyst was investigated and decreased surface acidity density was considered to cause the activity improvement. As the importance of basicity property was noticed, the relationship between activity and acidic–basicity was established. S. Loridant prepared different alkaline earth phosphates by co-precipitation and gas phase dehydration of lactic acid was evaluated.27 Correlation between acrylic selectivity of alkaline earth phosphates and the acid–base balance was clearly established. The AA selectivity was the highest for acid–base balance close to 1 and decreased significantly when this parameter increased. However, it was reported by Xu that for hydroxyapatites catalyst, the AA formation rate showed a volcano type dependence on this acidity–basicity ratio and the best ratio was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was different from Loridant.28 Quite recently, Jean-François Paul reported that lattice oxygen atoms could easily abstract a hydrogen atom from the methyl group of the lactic acid and this reaction led to carbanion formation with a lower activation energy than carbocation formation by direct C–OH breaking.29 The simulation results indicated that the basicity sites may facilitate a higher AA selectivity. In general, there is still some contradiction among all the conclusions and some new catalyst needs to be developed.
1, which was different from Loridant.28 Quite recently, Jean-François Paul reported that lattice oxygen atoms could easily abstract a hydrogen atom from the methyl group of the lactic acid and this reaction led to carbanion formation with a lower activation energy than carbocation formation by direct C–OH breaking.29 The simulation results indicated that the basicity sites may facilitate a higher AA selectivity. In general, there is still some contradiction among all the conclusions and some new catalyst needs to be developed.
In this paper, K2HPO4 and Al2(SO4)3 supported MCM-41 showed good AA selectivity and the reason was deduced from the acidic properties. In order to further investigate the relationship between structure and activity and to provide some guidance for catalyst design, some more catalysts with different loading amount and K2HPO4–Al2(SO4)3 ratio were developed. The catalysts were further examined with XRD, FTIR, CO2/NH3-TPD and the relationship between acid–basic property and selectivity was discussed.
2. Experimental
2.1. Materials
ML (analytic grade) was obtained from Sinopharm Chemical Reagent Co. and deionized water was used for its dilution. The alkali phosphates and sulphates were obtained from Sigma-Aldrich. MCM-41 was purchased from the Nankai University (China).2.2. Catalyst preparation
There were two steps for the two salts supported catalyst preparation. The first one was the impregnation with phosphate. 4 g of MCM-41 was added into 100 mL aqueous solution with a desired phosphate amount (1.2 g, 30 wt% of the support). The mixture was stirred at 80 °C for 3 h, and the resulting slurry was subjected to oven with air flow at 110 °C for 10 h, and the dried samples were then calcined at 550 °C for 3 h with a heating rate of 10 °C min−1. In the second step, the obtained catalyst from step one was added into 100 mL aqueous solution with a desired sulphate amount (0.8 g 20 wt% of the support). The mixture was stirred at 80 °C for 3 h, and the resulting slurry was subjected to oven with air flow at 110 °C for 10 h. The dried samples were calcined at 550 °C with a heating rate of 10 °C min−1 and then pressed at 20 MPa for 10 min and crushed into particles of 20–40 mesh.2.3. Catalyst evaluation
The evaluation of catalyst activity was carried out in an upright fixed-bed quartz reactor which was 8 mm in inner diameter and 300 mm in length. The reaction was operated at atmospheric pressure. A 2 g portion of catalyst was charged into the reactor, and the space above the catalyst bed was filled with quartz chips to ensure preheating of the in-coming ML-containing liquid. Before introduction of the feedstock, the sample was heated up in a flow of pure N2 (30 mL min−1) to a desired temperature at a rate of 10 °C min−1 and kept at this temperature for 0.5 h. Then a flow of LA liquid (1.2 mL h−1) was introduced, and the products were collected at a cold trap. The analysis of the collected species was conducted using a gas chromatograph equipped with FID and HP-FFAP capillary column (0.32 mm 25 m), and lactic acid butyl ester was adopted as internal standard.2.4. Catalysts characterization
Powder X-ray diffraction measurement was conducted on a Japan Science D/max-RB diffractometer employing Cu Kα radiation (λ = 0.15418 nm). The X-ray tube was operated at 45 kV and 150 mA. The specific surface areas and pore volumes of the catalysts were measured through nitrogen adsorption at 77 K using a Micrometrics ASAP 2020 instrument. Prior to adsorption, the samples were treated at 200 °C under vacuum for 3 h. The specific surface area was calculated according to the Brunauer–Emmett–Teller (BET) method.The basic–acid properties of the samples were evaluated by the temperature-programmed desorption (TPD) of CO2 and NH3. The TPD profiles were normalized by sample weight. The sample (0.1 g) in a glass U-tube was preheated at 773 K for 1 h in He flow (20 mL min−1), and then the catalyst was cooled from 773 K to room temperature. CO2 or NH3 (20 kPa) was introduced into the glass tube at room temperature for 30 min, and CO2 or NH3 was evacuated for 30 min at 373 K. The TPD measurements were conducted from 373 to 773 K at a heating rate of 10 K min−1.
The FTIR spectra of pyridine (CO2) adsorption were recorded in the range of 1000–4000 cm−1 on a Nicolet 6700 spectrometer. The samples were pressed into self-supporting wafers containing 20 mg of material, and then the wafers were mounted inside a Pyrex vacuum cell and degassed at 400 °C for 1 h. Samples were allowed to cool down to room temperature (RT), and pyridine vapor was admitted into the cell and adsorption lasted for 1 h.
3. Results
3.1. ML dehydration activity
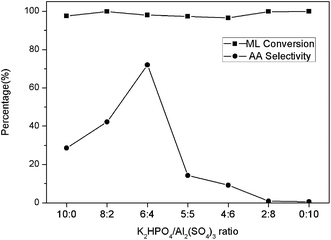
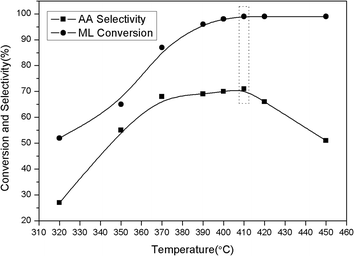
The catalytic activities of the phosphate and sulphate modified MCM-41 catalysts in ML conversions were listed in Table 1. The conversions of ML by these catalysts over the controlled reaction conditions gave mainly AA, MA, acetaldehyde and other small amounts of gaseous by-products such as carbon monoxide and hydrogen. It could be seen that the modification with both K2HPO4 and Al2(SO4)3 showed better AA selectivity compared with the single salt supported. Based on this, the effect of different K2HPO4 to Al2(SO4)3 ratio on catalyst activity was to be investigated. The K2HPO4 to Al2(SO4)3 ratio was chosen from 10 to 0 with the whole loading amount of 45 wt% and the results were shown in Fig. 1. Only the AA production was depicted since the amount of MA was too small compared with AA for each sample which were around 1–2%. For each ratio, the ML conversion was above 96% and there was a significant improvement in AA selectivity from the ratio 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 6
0 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Further decrease of the K2HPO4–Al2(SO4)3 ratio would result in a drop of AA selectivity. The selectivity to MA did not change remarkably within the whole range of the ratios. The highest AA selectivity could be obtained at the 6
4. Further decrease of the K2HPO4–Al2(SO4)3 ratio would result in a drop of AA selectivity. The selectivity to MA did not change remarkably within the whole range of the ratios. The highest AA selectivity could be obtained at the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio (K2HPO4–Al2(SO4)3).
4 ratio (K2HPO4–Al2(SO4)3).
| Catalyst | ML conversion (%) | Product selectivity | ||
|---|---|---|---|---|
| AA (%) | MA (%) | Acetaldehyde (%) | ||
| a Conditions: calcination temperature 550 °C, reaction temperature 400 °C, catalyst: 2 mL, particle size: 20–40 meshes, carrier gas N2: 30 mL min−1, feed flow rate: 1.2 mL h−1 ML feedstock: 60 wt% in water. | ||||
| MCM-41 | 99 | 1 | 0.2 | 70 |
| K2HPO4/MCM-41 | 93 | 43 | 2 | 45 |
| K2HPO4 + Al2(SO4)3/MCM-41 | 90 | 70 | 2 | 22 |
| Al2(SO4)3/MCM-41 | 95 | 0.8 | 0.1 | 72 |
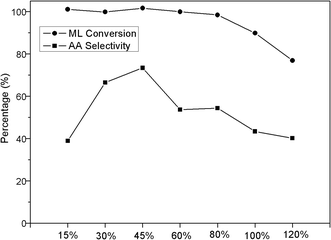
The effect of the two salts loading amount on catalyst activity was then investigated with the K2HPO4 to Al2(SO4)3 ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and the results were shown in Fig. 2. The ML conversion declined slowly as the loading amount increased in the range of 15–80 wt% and the conversions were all over 96%. When the loading amount was higher than 80%, there was a remarkable decrease of the conversion and the 120% loading sample showed the lowest conversion of 76%. A volcano type dependence appeared between the AA selectivity and the loading amount, and the highest AA selectivity was obtained at 45 wt% loading sample with the AA selectivity 72%. Further increase of the loading amount would result in a drop of AA selectivity.
4 and the results were shown in Fig. 2. The ML conversion declined slowly as the loading amount increased in the range of 15–80 wt% and the conversions were all over 96%. When the loading amount was higher than 80%, there was a remarkable decrease of the conversion and the 120% loading sample showed the lowest conversion of 76%. A volcano type dependence appeared between the AA selectivity and the loading amount, and the highest AA selectivity was obtained at 45 wt% loading sample with the AA selectivity 72%. Further increase of the loading amount would result in a drop of AA selectivity.
3.2. Catalyst characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
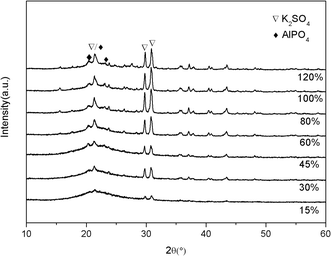
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The increase in the peak intensity could be related to the amount of K2SO4 and the increase of crystallinity, which needed further explore.
4. The increase in the peak intensity could be related to the amount of K2SO4 and the increase of crystallinity, which needed further explore.
As provided in Fig. 4, the effect of salts loading amounts on catalysts structure was investigated. As the loading amount increased from 15% to 80%, the peak intensities of K2SO4 and AlPO4 increased accordingly, indicating that more K2SO4 generated. However, there was no more increase when the loading amount was higher than 80%. Meanwhile, some more peaks such as the peaks centered at 18, 28° ascribed to some complex sulphate and phosphate appeared when the loading amount was higher than 45%. Combining the fact that the 45% loading sample showed the highest AA selectivity, it may indicate that the formation of complex sulphate and phosphate was detrimental for the selectivity. In other words, when the loading amount was 45%, the two precursors could completely react with each other and generate AlPO4 and K2SO4.
The BET surface area and pore structure of all samples were shown in Table 2. MCM-41 sample showed the largest surface area of 1003 m2 g−1 and the largest pore volume of 0.55 cm3 g−1. The introduction of two salts with any amount would cause a decrease of surface area and pore volume. As the two salts loading amount increased from 15% to 120%, the BET surface area and pore volume decreased accordingly.
| Catalyst | SBET (m2 g−1) | Pore volume (cm3 g−1) | Acid amount (μmol g−1) | Basic amount (μmol g−1) | A/B |
|---|---|---|---|---|---|
| 0% | 1003 | 0.55 | 720 | 266 | 2.7 |
| 15% | 287 | 0.398 | 164 | 441 | 0.27 |
| 45% | 48 | 0.29 | 86 | 45 | 1.91 |
| 120% | 49 | 0.266 | 140 | 24 | 5.83 |
Shown in Fig. 6 are the CO2-TPD profiles recorded over the two salts modified MCM-41 with different loadings. The unmodified MCM-41 sample showed three desorption peaks centered at 485, 643, and 676 °C and the basic amount was 266 μmol g−1. With the two salts addition at elevated loading, the 485 °C desorption peak diminished and only one peak in the range of 600–700 °C remained for all the modified samples. Based on the CO2-TPD result, the 15% loading amount sample showed the largest basic amount of 441 μmol g−1. There was a remarkable decrease of the basic density as the loading amount increased, and the 120% loading amount sample showed the smallest basic amount.
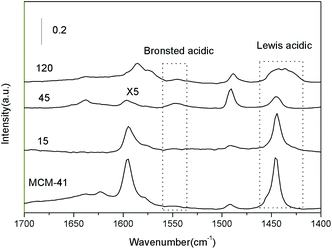
With the two salts supported together, the patterns varied with the loading amount. It could be seen that the 15% supported sample was similar to MCM-41. At higher loadings, the intensity of the 1440 cm−1 peak declined, suggesting that the Lewis acid sites associated with the framework of MCM-41 were blocked by the impregnation of two salts. To the 120% supported sample, it showed a broad peak around 1450 and 1600 cm−1, indicating that some new Lewis acids associated with the two salts appeared besides the Lewis acid related to MCM-41. It should be pointed out that the bands intensities of 45% supported sample were really low compared with all the other samples, indicating that this sample had the smallest surface acid sites. This was consistent with the NH3-TPD results.
3.3. Catalytic activity optimization
| LHSV (h−1) | ML Conversion (%) | AA selectivity (%) | Acetaldehyde (%) | AA yield (%) |
|---|---|---|---|---|
| a Conditions: temperature: 410 °C, catalyst: 2 mL, particle size: 20–40 meshes, carrier gas N2: 30 mL min−1, ML feedstock: 60 wt% in water. K2HPO4–AL2(SO4)3/MCM-41 (45 wt%). | ||||
| 0.2 | 98.1 | 59.2 | 15.9 | 58.1 |
| 0.4 | 99.8 | 62.3 | 17.5 | 62.2 |
| 0.6 | 98.5 | 70.8 | 16.0 | 70.0 |
| 1 | 95.2 | 66.6 | 15.9 | 63.4 |
| 1.5 | 83.5 | 64.8 | 14.8 | 54.1 |
| 2.5 | 59.9 | 63.3 | 13.4 | 37.9 |
| ML concentration (%) | ML conversion (%) | AA selectivity (%) | Acetaldehyde (%) | AA yield (%) |
|---|---|---|---|---|
| a Conditions: temperature: 410 °C, catalyst: 2 mL, particle size: 20–40 meshes, carrier gas N2: 30 mL min−1, feed flow rate: 1.2 mL h−1 K2HPO4–AL2(SO4)3/MCM-41 (45 wt%). | ||||
| 15 | 98.6 | 56.1 | 22 | 55.3 |
| 30 | 98.5 | 59.2 | 21 | 58.3 |
| 45 | 98.5 | 65.1 | 19 | 64.1 |
| 60 | 98.5 | 70.8 | 17 | 70.0 |
| 75 | 98.0 | 68.3 | 19 | 66.9 |
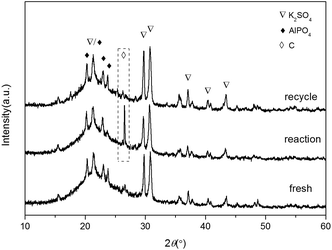
In order to investigate the reason for the catalyst deactivation, the fresh and used catalyst and the regenerated sample were examined with XRD (Fig. 11). It could be seen that the main peaks of the three samples were quite similar except the new peak centered at 26.6° belonging to graphite appeared for the reaction one. After the regeneration, the graphite peak disappeared and the profile became the same as the fresh one, indicating that the catalyst could be totally regenerated. Besides, it was proved that the dehydration reaction and the regeneration process had little effect on the catalyst structure, indicating that the samples could be promising industrial catalysts with its good stability.
4. Discussion
It has been known that the catalytic dehydration rate of alcohol over catalyst would be dependent on the catalyst surface acid–base property. An attempt is made to correlate the rates of ML conversion and AA formation with the surface acidity and basicity of the modified catalysts. The acidic amounts and basicity amounts calculated by NH3(CO2)-TPD as well as the ratio between acidic and basicity were shown in Table 1. MCM-41 showed the lowest AA selectivity due to its strong acidic sites. For the other three samples, the AA selectivity was negatively related to the acidic density and 45% sample showed the lowest acidic density and highest AA selectivity. Basic sites should play an important role in the dehydration reaction since it can dissociate H–C bond and enhance the dehydration reaction. In our study, the best AA selectivity appeared when the acid–basic = 1.91 on the 45% sample, which was different from the result of Xu (acidic–basicity = 4) and Loridant (acidic–basicity = 1). The difference may come from different catalysts and the precursors between LA and ML. In general, specific to this catalyst, the acid–basic ≈ 1.9 was best for the AA selectivity. The best acidic–basicity ratio varied on catalysts may attribute to the ML adsorption or consecutive reactions.5. Conclusion
By modifying the loading amount and K2HPO4–Al2(SO4)3 ratio, the surface acid–basic properties of MCM-41 based catalyst could be adjusted and used in ML dehydration reaction. 72% AA selectivity was achieved over 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of K2HPO4 and Al2(SO4)3 and 45 wt% supported MCM-41 catalyst. The higher selectivity for acrylic acid should be correlated to the synergistic effect of the weaker acid site density and moderate basic site density as well as the acidic–basicity ratio. The catalyst showed a good stability and could be regenerated after air treatment.
4 ratio of K2HPO4 and Al2(SO4)3 and 45 wt% supported MCM-41 catalyst. The higher selectivity for acrylic acid should be correlated to the synergistic effect of the weaker acid site density and moderate basic site density as well as the acidic–basicity ratio. The catalyst showed a good stability and could be regenerated after air treatment.
Acknowledgements
The authors would like to acknowledge the Ministry of Science and Technology, PR China for the financial support of Project 2013CB733600. We would also thank China Postdoctoral Science Foundation (2013M530515).References
- L. F. Boesel and R. L. Reis, Prog. Polym. Sci., 2008, 33, 180–190 CrossRef CAS PubMed.
- C. M. Tang, Y. Zeng, X. G. Yang, Y. C. Lei and G. Y. Wang, J. Mol. Catal. A: Chem., 2009, 314, 15–20 CrossRef CAS PubMed.
- X. Xu, J. Lin and P. Cen, Chin. J. Chem. Eng., 2006, 14, 419–427 CrossRef CAS.
- V. Grabovac, D. Guggi and A. B. Schnürch, Adv. Drug Delivery Rev., 2005, 57, 1713–1723 CrossRef CAS PubMed.
- M. M. Lin, Appl. Catal., A, 2001, 207, 1–16 CrossRef CAS.
- X. J. Yang, R. M. Feng, W. J. Ji and C. T. Au, J. Catal., 2008, 253, 57–65 CrossRef CAS PubMed.
- M. M. Lin, Appl. Catal., A, 2003, 250, 305–318 CrossRef CAS.
- P. Botella, J. M. L. Nieto, B. Solsona, A. Mifsud and F. Márquez, J. Catal., 2002, 209, 445–455 CrossRef CAS.
- X. Tong, Y. Ma and Y. Li, Appl. Catal., A, 2010, 385, 1–13 CrossRef CAS PubMed.
- P. Sun, D. H. Yu, Z. C. Tang, H. Li and H. Huang, Ind. Eng. Chem. Res., 2010, 49, 9082–9087 CrossRef CAS.
- P. Sun, D. H. Yu, K. M. Fu, M. Y. Gu, Y. Wang, H. Huang and H. H. Ying, Catal. Commun., 2009, 10, 1345–1349 CrossRef CAS PubMed.
- M. H. Gao, M. Hiratu, E. Toorisaka and T. Hano, Bioresour. Technol., 2006, 97, 2414–2420 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, Biotechnol. Adv., 2013, 31, 877–902 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, J. Biotechnol., 2011, 156, 286–301 CrossRef CAS PubMed.
- M. Jarvinen, L. Myllykoski, R. Keiski and J. Sohlo, Bioseparation, 2000, 9, 163–166 CrossRef CAS.
- Y. Fan, C. Zhou and X. Zhu, Catal. Rev., 2009, 51, 293–324 CAS.
- Y. Matsuura, A. Onda, S. Ogo and K. Yanagisawa, Catal. Today, 2014, 226, 192–197 CrossRef CAS PubMed.
- Y. Matsuura, A. Onda and K. Yanagisawa, Catal. Commun., 2014, 48, 5–10 CrossRef CAS PubMed.
- C. M. Tang, J. S. Peng, G. C. Fan, X. L. Li, X. L. Pu and W. Bai, Catal. Commun., 2014, 43, 231–234 CrossRef CAS PubMed.
- J. Yan, D. H. Yu, P. Sun and H. Huang, Chin. J. Catal., 2011, 32, 405–411 CrossRef CAS.
- J. Yan, D. H. Yu, H. Li, P. Sun and H. Huang, J. Rare Earths, 2010, 28, 803–806 CrossRef CAS.
- J. F. Zhang, Y. L. Zhao, M. Pan, X. Z. Feng, W. J. Ji and C. T. Au, ACS Catal., 2011, 1, 32–41 CrossRef CAS.
- V. C. Ghantani, S. T. Lomate, M. K. Dongare and S. B. Umbarkar, Green Chem., 2013, 15, 1211–1217 RSC.
- J. F. Zhang, Y. L. Zhao, X. Z. Feng, M. Pan, J. Zhao, W. J. Ji and C. T. Au, Catal. Sci. Technol., 2014, 4, 1376–1385 CAS.
- J. Peng, X. Li, C. Tang and W. Bai, Green Chem., 2014, 16, 108–111 RSC.
- H. J. Wang, D. H. Yu, P. Sun, J. Yan, Y. Wang and H. Huang, Catal. Commun., 2008, 9, 1799–1803 CrossRef CAS PubMed.
- E. Blanco, P. Delichere, J. M. M. Millet and S. Loridant, Catal. Today, 2014, 226, 185–191 CrossRef CAS PubMed.
- B. Yan, L. Z. Tao, Y. Liang and B. Q. Xu, ACS Catal., 2014, 4, 1931–1943 CrossRef CAS.
- C. Hammaecher and J. F. Paul, J. Catal., 2013, 300, 174–182 CrossRef CAS PubMed.
- B. Chakraborty and B. Viswanathan, Catal. Today, 1999, 49, 253–260 CrossRef CAS.
- X. Shao, X. Zhang, W. Yu, Y. Wu, Y. Qin, Z. Sun and L. Song, Appl. Surf. Sci., 2012, 263, 1–7 CrossRef CAS PubMed.
- T. D. Conesa, J. M. Hidalgo, R. Luque, J. M. Campelo and A. A. Romero, Appl. Catal., A, 2006, 299, 224–234 CrossRef CAS PubMed.
- C. Cai, H. Wang and J. Han, Appl. Surf. Sci., 2011, 257, 9802–9808 CrossRef CAS PubMed.
- F. Wang, N. Ta and W. J. Shen, Appl. Catal., A, 2014, 475, 76–81 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |