Mesoporous silica-coated luminescent Eu3+ doped GdVO4 nanoparticles for multimodal imaging and drug delivery†
Abstract
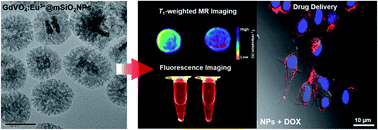
We describe a simple method for synthesizing mesoporous silica-coated luminescent europium-doped gadolinium vanadate (GdVO4:Eu3+@mSiO2) nanoparticles. Their biomedical applications as a potential imaging nanoprobe for both fluorescence imaging and magnetic resonance imaging (MRI) and as a simultaneous anti-cancer drug delivery vehicle are also discussed. Eu3+ doped GdVO4 nanoparticles exhibit strong red photoluminescence and the Gd3+ in GdVO4 can be used as a T1 contrast agent for MRI. T1-weighted MR contrast enhancement as well as sustained intracellular drug delivery can be achieved by the mesoporous silica layer coating onto a single nanoparticle. Enhanced T1 MR contrast was proven by relaxometric studies on water access to the paramagnetic core. GdVO4:Eu3+@mSiO2 nanoparticles as a new type of theragnostic (imaging and treatment) agent can provide new opportunities in a cancer treatment.

 Please wait while we load your content...
Please wait while we load your content...