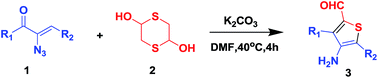
Facile preparation of 3,5-disubstituted-4-aminothiophene-2-carbaldehyde from a novel unexpected domino reaction of vinyl azides and 1,4-dithiane-2,5-diol†
Abstract
A simple and direct synthesis of 3,5-disubstituted-4-aminothiophene-2-carbaldehyde from vinyl azides and 1,4-dithiane-2,5-diol was developed. An attractive feature of this protocol is that the desired products are generated in a highly efficient and eco-friendly manner. A plausible mechanism has been proposed.

 Please wait while we load your content...
Please wait while we load your content...