Ag/Cu co-doped ZnS–In2S3 solid solutions: facile synthesis, theoretical calculations and enhanced photocatalytic activity†
Jingxue Suna,
Gang Chen*a,
Yujie Feng*b and
Yu Wanga
aDepartment of Chemistry, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: gchen@hit.edu.cn
bState Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: yujief@hit.edu.cn
First published on 28th August 2014
Abstract
ZnS–In2S3 nanospheres were prepared by a simple hydrothermal method without using any organic solvents or templates. In order to improve the photocatalytic performance, Ag and Cu were chosen as doping elements for both single-doping and co-doping. The band structure, electronic structure and electron density were carefully investigated based on density functional theory (DFT). The roles of In and Ag/Cu in the solid solutions are found to be band adjustment, electron density control and electron mobility optimization due to the d orbitals of Ag and Cu. A series of photocatalysts were further characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), UV-visible diffuse reflectance spectra (UV-vis) and Brunauer–Emmett–Teller (BET) surface area measurement. It is found that the formation of a solid solution greatly broadened the light response range and made visible light response possible. When the amounts of Ag and Cu are 0.2 mL and 0.3 mL, the Ag/Cu co-doped ZnS–In2S3 solid solution showed the optimized value of 2.15 mmol h−1 g−1 under visible-light irradiation without using any noble metal as a co-catalyst. After four cycles of photocatalytic tests, the activity barely decreased.
Introduction
H2 evolution via water splitting with renewable energy sources is a fascinating strategy from the view point of environmentally clean energy for the future. Among the H2 evolution methods, photocatalytic water splitting using semiconductors as photocatalysts has attracted wide attention since reported in 1972 and has been considered as one of the most growth potential ultimate solutions to energy and environmental issues.1–3 Among the various kinds of photocatalyst oxide, especially TiO2, is most widely studied because of its chemical stability, nontoxicity and high photocatalytic activity.4–6 However, some shortcomings such as low solar efficiency hinder its extensive application. Therefore, one key route for H2 evolution is water splitting using semiconductor photocatalysts under solar light. Among the metallic oxides, most of their valance bands consist of O 2p orbitals, which are relatively lower than the position of water oxidation. Compared to metallic oxide, sulfides, which have narrow band gaps (BGs) and valence bands at relatively negative potentials compared to oxides, can be used as good candidates for visible-light-driven photocatalysts due to the hybridization of O 2p and S 3p.7–12Till now, in order to adjust the bandgap of photocatalysts for the purpose of extending the absorption of light into the visible region, three main approaches have been widely used: (I) modification of the VB (valence band), (II) adjustment of the CB (conduction band), and (III) continuous modulation of the VB and/or CB.13,14 Cation doping and anion doping are the most widely used method for the VB and CB adjustment. In recent years, sulfide solid solutions have been extensively studied because of their continuous modulation of the VB and CB without introducing impurity levels and excellent performance for photocatalytic hydrogen production under visible light irradiation, such as ZnS–CdS,8,11,15–17 ZnS–AgInS2,18–20 ZnS–CuInS2 (ref. 21 and 22) and so on. Recently, we have reported that metal ions with d orbitals are beneficial to the narrowing of bandgap, such as Ag and Cu.23,24 Furthermore, to understand the structural and electronic properties of solid solutions, theoretical studies are desirable.25–27 Few theoretical papers on solid solutions have appeared in the literature until recently.
In this work Ag and Cu are chose as co-doping element to adjust the bandgap of ZnS–In2S3 solid solution and standard density functional theory (DFT) calculations are also performed to study the influences of co-doping on band structures and electronic structures.
Experimental
Catalysts preparation
In a typical process, Zn(Ac)2·2H2O (0.6 mmol), InCl3·4H2O (0.1 mmol), and thioacetamide (TAA) (2.4 mmol) were dissolved into 7.8 mL of pure water under magnetic stirring to form a clear solution (Solution A). AgNO3 or Cu(Ac)2·2H2O are chose as Ag or Cu source and are prepared to aqueous solution of 0.025 M (Solution B). Then Solution B was added dropwise into Solution A and then immediately transferred into an autoclave (Teflon cups with 15 mL inner volume). The autoclave was kept in the oven under 180 °C for 18 h and the cooled to room temperature. The product was collected by centrifugation, washed several times with absolute ethanol, and finally air dried.Characterization
The structure of solid-solutions was analyzed by X-ray diffraction (XRD) on Rigaku D/max-2000 diffractometer (Cu Kα λ = 0.15406 nm). The morphology of the as-prepared samples was observed on FEI Quanta 200F field emission scanning electron microscope (FE-SEM). UV-vis diffuse reflectance spectra were acquired by a spectrophotometer (TU-1900) and BaSO4 was used as the reflectance standard.The photocatalytic hydrogen generation test was performed in a gas-closed circulation system with a side quartz window. The as prepared powder was pre-dispersed by ultrasonication (100 W) for 30 min in an aqueous solution (320 mL) containing Na2SO3 (1.2 mol L−1) and Na2S (0.1 mol L−1) as electron donors. The reaction was carried out by irradiating the mixture with light from a Xe lamp (Trusttech PLS-SXE 300 W, Beijing) which was equipped with an optical filter (λ > 400 nm) to cut off the light in the ultraviolet region. The amount of produced H2 was measured by gas chromatography (Agilent 6820) with a thermal conductivity detector (TCD) and Ar as the carrier gas.
Calculation method
The plane-wave pseudopotential method, based on density functional theory (DFT), was used to calculate the electronic structures for Ag/Cu co-doped solid solution by employing the CASTEP program,28 The generalized gradient approximation (GGA) in the scheme of Perdew–Burke–Ernzerhof (PBE) was used to describe the electronic exchange–correlation interactions29 that is known to be an efficient and accurate scheme for solving the many-electron problem of a crystal.30 The density of the Monkhorst-Pack k-point mesh are 6 × 6 × 6. The absorption curves can be obtained from the imaginary part of the dielectric constant from DFT calculation. The kinetic energy cut-off was set at 310 eV, and the geometric structures were first optimized by first-principle calculations. The Ag and Cu atom are substitute for zinc atoms in the crystal structure. The concentration of metal dopant was about 8%, comparable to the experimental value of 2%. During the structural optimizations, the cell shape and the positions of all atoms were allowed to be relaxed at an energy cutoff of 400 eV.Results and discussion
Crystal structure
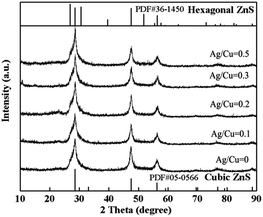
Fig. 1 shows the XRD patterns of ZnS–In2S3 solid solutions with different doping amount of Ag/Cu, which are denoted as Z-0, Z-0.1, Z-0.2, Z-0.3 and Z-0.5. As shown, the relatively broader peaks in pattern are attributed to the absence of long-range order in these samples and reflect the small particle size (ca. 20 nm from the Scherrer eqn (1)).
D = Kλ/(B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
Morphology
After hydrothermal treatment for 18 h under 180 °C, the morphology of products are investigated by FE-SEM characterization, as shown in Fig. 2. From the overall morphology of ZnS–In2S3 (Fig. 2a), we can indicate that the sample is composed of numerous nearly-monodispersed spheres. The diameter of the spheres is around 200 nm (150–200 nm). Further observation (Fig. 2b) shows that the sphere consists of numerous tiny particles with the size of tens of nanometers. The rough surface can provide more active sites which is benefit for the photocatalytic process. When Ag/Cu is doped into the ZnS–In2S3, the shape of the products remains to be spheres, while the size is obviously decreased. As shown in Fig. 2c and d, the diameter of the sphere is 50–100 nm. As a result of the smaller size, the Brunauer–Emmett–Teller (BET) surface area is up to 76 m2 g−1, which is much lager than pure ZnS (43 m2 g−1) and ZnS–In2S3 (14 m2 g−1). Meanwhile, the lager surface area means the more sufficient contact of the photocatalyst and the reaction agent. | ||
| Fig. 2 FE-SEM images of (a and b) ZnS–In2S3 solid solution and (c and d) Ag/Cu do-doped ZnS–In2S3 solid solution. | ||
UV-vis diffuse reflectance spectra
Light absorbing properties are of importance for photocatalysts24,31 because the substitution of atoms into the lattice may result in noteworthy influence on the visible light responding. The UV-vis diffuse reflectance spectra of the samples with different amount of Ag/Cu co-doping are shown in Fig. 3. The absorption edge of the ZnS–In2S3 is at 393 nm, corresponding to the bandgap of 3.15 eV, which is in UV light region. The steep edge also indicates that the absorption relevant to the bandgap is related to the intrinsic transition of the semiconductor and not to the transition from impurity levels.23,32 When Ag/Cu is doped into the lattice, the color of the product changes from white to light yellow, and the absorbance edge shows red-shift. Further increasing the doping amount, the absorption wavelength extended into the longer wavelength region, from 393 nm to 540 nm (Ag/Cu = 0.5 mL). According the formula (2), the bandgap energy of Z-0, Z-0.1, Z-0.2, Z-0.3 and Z-0.5 are 3.15 eV, 2.98 eV, 2.71 eV, 2.53 eV and 2.30 eV, respectively.| Eg (eV) = 1240 (nm)/λ (nm) | (2) |
Eg stands for the bandgap energy, λ stands for the light absorbance edge. The absorption shoulder of the diffuse reflectance spectra indicates that continuous levels are formed by the dopants in the forbidden band, which needs to be proved by theoretical calculations.
Photocatalytic activities
The effect on the photocatalytic activity of Ag/Cu co-doping on ZnS–In2S3 solid solution samples prepared was systematically investigated. Fig. 4 shows the dependence of photocatalytic activity on different amount of Ag/Cu. As shown, when no Ag or Cu is doped, the rate of hydrogen evolution is only 0.2 mmol h−1 g−1. When Ag is doped into the lattice, the rate shows obviously increasing (1.4 mmol h−1 g−1 for Ag = 0.1 mL and 1.65 mmol h−1 g−1 for Ag = 0.2 mL) under visible light irradiation (λ > 400 nm). It is also worth noting that when the amount of Ag further increases, the photocatalytic activity shows a trend of declining. The reason is suggested to be the recombined center induced by Ag. When Ag and Cu are co-doped into the lattice of the solid solution, the rate of photo-generated hydrogen is further improved compared with only Ag doping. As can be seen, when the amount of Ag and Cu is 0.2 mL and 0.3 mL, the maximum photocatalytic activity is achieved. The highest rate is calculated to be 2.15 mmol h−1 g−1, which is more than ten times of ZnS–In2S3 solid solution. This means the synactic effects of Ag and Cu is benefit for the improving of photocatalysis.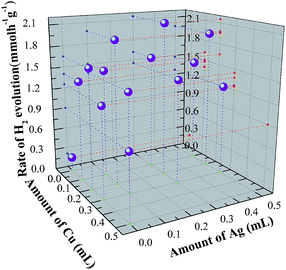 | ||
| Fig. 4 Rates of photocatalytic hydrogen evolution of the samples with different amount of Ag/Cu co-doping. | ||
In order to investigate the photo-stability of optimized photocatalyst Ag/Cu co-doped ZnS–In2S3 solid solution (Ag = 0.2 mL and Cu = 0.3 mL), a typical reaction time course for hydrogen evolution from an aqueous Na2SO3/Na2S solution under visible light irradiation is carried out.33 As shown in Fig. S2,† four runs were taken out and the reaction system was evacuated after each run. During the first run, the average hydrogen evolution rate can reach up to 2.05 mmol h−1 g−1. During the fourth run, the average rate is 1.85 mmol h−1 g−1, which means the photocatalyst shows good photo-stability under visible light irradiation. The decrease in the rate of hydrogen evolution might be related to the deactivation of the photocatalyst or attributed to the consumption of the sacrificial reagents in the solution.
Based on the previous report, the improved performances of photocatalysis may due to the following reasons: (1) large surface area which leads to more sufficient contact and more active sites;34 (2) small particle size which leads to short migration time of photo-induced carriers;35 (3) appropriate band structure. Usually, large bandgap energy makes photocatalyst powerless in visible light responding and small bandgap energy can improve the ability of visible light responding. Meanwhile, too small band gap energy may cause the weak reducing ability of photo-generated electrons, which is adverse for the photocatalysis;36 (4) appropriate electron delocalization which means the favorable migration environment of photo-induced carriers.37 Based on the above characterization and analysis, the obvious improvement of photocatalysis can attribute to reason (1) and (2). The effects of the latter two reasons are then further calculated by theoretical methods.
Band structures and electron density
Band structures of ZnS and Ag/Cu co-doped ZnS–In2S3 were calculated based on density functional theory (DFT) in order to reveal the effects of In3+, Cu2+ and Ag+ on the band structure of ZnS. Fig. S1a and S1b† (ESI) show the band structure of ZnS and Ag/Cu co-doped ZnS–In2S3, respectively. As can be seen, the top of the valance bands and the bottom of the conductive bands of both samples locate at G point, indicating that both compounds are direct band gap semiconductors, which is benefit for photocatalysts, also accordance with the UV-vis analysis. Fig. 5 shows the density of states (DOS) and partial density of states (PDOS). As shown in Fig. 5a, both of the conduction and valence bands of ZnS consisted of the hybrid orbitals of Zn and S due to the covalency. The conduction band of ZnS consists of Zn 4s4p orbitals and S 3p orbital contributes to the valance band of ZnS, similar as the results reported.12,38,39 The bandgap energy calculated from the band structure and DOS is 2.4 eV, which is smaller that the bandgap energy obtained from the UV-vis analysis. This is a common feature of DFT calculations,40 also an artifact of the GGA method used for this calculation.33,41 For Ag/Cu co-doped ZnS–In2S3, as shown in Fig. 5b, the bandgap energy is reduced to 1.25 eV. The narrower bandgap is due the hybridized orbital induced by In, Ag and Cu. As shown in Fig. 5b, the bottom of CB moves to low energy level due to the hybridization of Zn d orbital and In d orbital, while the In d orbital barely contribute to the top of VB. The top of valance band moves to high energy which because the introducing of d orbitals of Ag and Cu. Therefore, the band gap is narrowed due to the formation of hybridized orbitals, as described in Fig. 6.Furthermore, the conduction band is broadened after the introducing of In, Ag and Cu, meaning that the delocalization of electron is improved. The broadened conduction band is consist of In 5s5p and Zn 4s4p, indicating that the broadened phenomenon is mainly due to the effect of In, while the d orbitals of Ag and Cu attribute to the shift of the valance band. Meanwhile, the stepwised red-shifting of the absorbance edge and continuous CB bottom and VB top further confirmed the formation of solid solution. Thus can be seen, the changing of H2 production rate on the Ag/Cu could be due to the tailoring of band structure and light absorbance. When Ag/Cu was induced into the lattice, the band gap is reduced due to the continuous expanding of VB, which is accordance with the UV-vis spectra analysis in Fig. 3.
The band gap energy mainly contributes to the light absorbance of the photocatalysts, while the electron density also has an effect on the activity of photocatalyst and is useful to discuss the origin of the narrow band gap of visible-light responsive photocatalysts. Plotted electron densities around cation atoms are shown in Fig. 7. As shown, when In and Ag/Cu was present in the crystal lattice of ZnS, this type of impurity plays the role of donor energy level. The co-doped solid solution has more electron-carriers compared with pure ZnS. Also, the CB width is obviously broadened. Furthermore, except for carriers, the electron delocalization is proved, which is also favorable for the photo-induced electron transportation. Therefore, this type of doping could obviously enhance the photocatalytic performance of under visible-light irradiation.
Conclusions
In summary, ZnS–In2S3 solid solution was synthesized by a simple hydrothermal method without using any organic templates or solvents. The formation of solid solution greatly broadened the light response range and made it possible for visible light responding. The method of Ag/Cu co-doping into the solid solution further improved the photocatalytic performances for hydrogen evolution. Theoretical calculation indicated that the incorporation of Ag/Cu expanded the VB and adjusted the electron density. The enhance activity is supposed to be due to the large surface area, small particle size and appropriate band structure and electron density. When the amount of Ag and Cu is 0.2 mL and 0.3 mL, the maximum photocatalytic activity is achieved (2.15 mmol h−1 g−1), which was more than ten times of ZnS–In2S3 solid solution. After four cycles of photocatalytic tests, the activity showed barely decreasing.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (21271055). We acknowledge for the support by Fundamental Research Funds for the Central Universities (HIT. IBRSEM. A.201410), Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (no. QAK201304) and Program for Innovation Research of Science in Harbin Institute of Technology (B201412).Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS.
- Y. P. Li, J. Zhan, L. Y. Huang, H. Xu, H. M. Li, R. X. Zhang and S. L. Wu, RSC Adv., 2014, 4, 11831–11839 RSC.
- T. N. He, X. L. Guo, K. Zhang, Y. M. Feng and X. D. Wang, RSC Adv., 2014, 4, 5880–5886 RSC.
- S. Sakulkhaemaruethai and T. Sreethawong, Int. J. Hydrogen Energy, 2011, 36, 6553–6559 CrossRef CAS PubMed.
- R. Zhao, R. F. Ding, S. J. Yuan, W. Jiang and B. Liang, Int. J. Hydrogen Energy, 2011, 36, 1066–1073 CrossRef CAS PubMed.
- J. X. Sun, G. Chen, Y. X. Li, R. C. Jin and Q. Wang, Energy Environ. Sci., 2011, 4, 4052–4060 CAS.
- X. W. Wang, G. Liu, L. Z. Wang, Z. G. Chen, G. Q. Lu and H. M. Cheng, Adv. Energy Mater., 2012, 2, 42–46 CrossRef.
- S. A. Macias-Sanchez, R. Nava, V. Hernandez-Morales, Y. J. Acosta-Silva, L. Gomez-Herrera, B. Pawelec, S. M. Al-Zahrani, R. M. Navarro and J. L. G. Fierro, Int. J. Hydrogen Energy, 2012, 37, 9948–9958 CrossRef CAS PubMed.
- F. Z. Jia, Z. P. Yao and Z. H. Jiang, Int. J. Hydrogen Energy, 2012, 37, 3048–3055 CrossRef CAS PubMed.
- D. Barpuzary, Z. Khan, N. Vinothkumar, M. De and M. Qureshi, J. Phys. Chem. C, 2012, 116, 150–156 CAS.
- X. Xu, R. J. Lu, X. F. Zhao, S. L. Xu, X. D. Lei, F. Z. Zhang and D. G. Evans, Appl. Catal., B, 2011, 102, 147–156 CrossRef CAS PubMed.
- N. S. Chaudhari, S. S. Warule and B. B. Kale, RSC Adv., 2014, 4, 12182–12187 RSC.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- M. Pal, S. Bera, S. Sarkar and S. Jana, RSC Adv., 2014, 4, 11552–11563 RSC.
- K. Ikeue, S. Shiiba and M. Machida, ChemSusChem, 2011, 4, 269–273 CAS.
- M. C. Liu, L. Z. Wang, G. Q. Lu, X. D. Yao and L. J. Guo, Energy Environ. Sci., 2011, 4, 1372–1378 CAS.
- K. Zhang, D. W. Jing, Q. Y. Chen and L. J. Guo, Int. J. Hydrogen Energy, 2010, 35, 2048–2057 CrossRef CAS PubMed.
- S. J. Zhang, RSC Adv., 2014, 4, 15835–15840 RSC.
- H. Xu, J. X. Zhu, Y. X. Song, W. K. Zhao, Y. G. Xu, Y. H. Song, H. Y. Ji and H. M. Li, RSC Adv., 2014, 4, 9139–9147 RSC.
- T. A. Arun, D. K. Chacko, A. A. Madhavan, T. G. Deepak, G. S. Anjusree, T. Sara, S. Ramakrishna, S. V. Nair and A. S. Nair, RSC Adv., 2014, 4, 1421–1424 RSC.
- W. J. Zhang and X. H. Zhong, Inorg. Chem., 2011, 50, 4065–4072 CrossRef CAS PubMed.
- H. W. Huang, S. B. Wang, N. Tian and Y. H. Zhang, RSC Adv., 2014, 4, 5561–5567 RSC.
- Y. X. Li, G. Chen, Q. Wang, X. Wang, A. K. Zhou and Z. Y. Shen, Adv. Funct. Mater., 2010, 20, 3390–3398 CrossRef CAS.
- Y. X. Li, G. Chen, C. Zhou and J. X. Sun, Chem. Commun., 2009, 2020–2022 RSC.
- H. S. Park, K. E. Kweon, H. Ye, E. Paek, G. S. Hwang and A. J. Bard, J. Phys. Chem. C, 2011, 115, 17870–17879 CAS.
- S. Kawasaki, K. Akagi, K. Nakatsuji, S. Yamamoto, I. Matsuda, Y. Harada, J. Yoshinobu, F. Komori, R. Takahashi, M. Lippmaa, C. Sakai, H. Niwa, M. Oshima, K. Iwashina and A. Kudo, J. Phys. Chem. C, 2012, 116, 24445–24448 CAS.
- J. Nisar, B. C. Wang, C. M. Araujo, A. F. da Silva, T. W. Kang and R. Ahuja, Int. J. Hydrogen Energy, 2012, 37, 3014–3018 CrossRef CAS PubMed.
- O. Jepsen, O. K. Andersen and A. R. Mackintosh, Phys. Rev. B: Condens. Matter Mater. Phys., 1975, 13, 3060–3083 Search PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, B864–871 CrossRef.
- Y. X. Li, G. Chen, H. J. Zhang, Z. H. Li and J. X. Sun, J. Solid State Chem., 2008, 181, 2653–2659 CrossRef CAS PubMed.
- H. X. Zhao, H. T. Yu, X. Quan, S. Chen, H. M. Zhao and H. Wang, RSC Adv., 2014, 4, 624–628 RSC.
- H. L. Hou, L. Wang, F. M. Gao, G. D. Wei, J. J. Zheng, B. Tang and W. Y. Yang, RSC Adv., 2014, 4, 19939–19944 RSC.
- Z. W. Luo, H. Jiang, D. Li, L. Z. Hu, W. H. Geng, P. Wei and P. K. Ouyang, RSC Adv., 2014, 4, 17797–17804 RSC.
- H. Kato, K. Asakura and A. Kudo, J. Am. Chem. Soc., 2003, 125, 3082–3089 CrossRef CAS PubMed.
- M. Xu, J. T. Zai, Y. P. Yuan and X. F. Qian, J. Mater. Chem., 2012, 22, 23929–23934 RSC.
- B. Kumar, S. Saha, A. Ganguly and A. K. Ganguli, RSC Adv., 2014, 4, 12043–12049 RSC.
- Y. D. Liu, L. Ren, X. Qi, Y. Wang, X. J. Liu and J. X. Zhong, RSC Adv., 2014, 4, 8772–8778 RSC.
- B. Weng, S. Q. Liu, Z. R. Tang and Y. J. Xu, RSC Adv., 2014, 4, 12685–12700 RSC.
- Y. X. Li, G. Chen, H. J. Zhang and Z. H. Li, Mater. Res. Bull., 2009, 44, 741–746 CrossRef CAS PubMed.
- J. W. Liu, G. Chen, Z. H. Li and Z. G. Zhang, J. Solid State Chem., 2006, 179, 3704–3708 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05960c |
| This journal is © The Royal Society of Chemistry 2014 |