Two stage chemical bath deposition of MoO3 nanorod films
Abstract
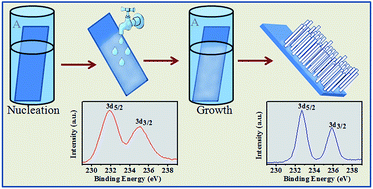
Thin films of hexagonal MoO3 nanorods are deposited by chemical bath deposition from a highly acidic aqueous solution containing ammonium heptamolybdate at 85 °C. We present a unique two-stage deposition process that results in uniform film formation even from a highly depleted solution. Growth occurs during the second stage only if the substrate removed from the first stage solution is first rinsed with water. Mechanistically, the proposed process is a combination of formation of nucleation sites at the first stage, removal or modification of a passivation layer on the nucleation sites by the rinse and chemical heterogeneity-induced growth at the second stage. Variations in either of these two deposition stages lead to control over rod dimensions and film texture. An FTIR study confirms the presence of amine and hydroxyl groups tightly bound in the crystals. Furthermore, h-MoO3 nanorod films showed good photocatalytic activity towards degradation of methylene blue (MB) under visible light.

 Please wait while we load your content...
Please wait while we load your content...