Synthesis and characterisation of layered double hydroxide dispersions in organic solvents†
Miaosen Yang,
Olivia McDermott,
Jean-Charles Buffet and
Dermot O'Hare*
Chemistry Research laboratory, 12 Mansfield Road, OX1 3TA Oxford, UK. E-mail: Dermot.ohare@chem.ox.ac.uk
First published on 6th October 2014
Abstract
Aqueous Miscible Organic Solvent Treatment (AMOST) Mg3AlCO3–LDHs have been prepared using twelve different (AMO) solvents. We find that the AMOST process produces significant changes to the crystallinity, morphology, thermal behaviour and dramatically increases the surface areas of the LDHs compared to conventional LDHs. Remarkably AMO–LDHs platelets are now hydrophobic and can be dispersed in a range of organic liquids. Clear dispersions of AMO–Mg3AlCO3–LDHs are observed in aromatic solvents. Overall, we found that the optimum AMO–Mg3AlCO3–LDHs organic solvent dispersions are obtained using 1-propanol as a treating solvent and ethyl benzene as the dispersion solvent. This system produces a monophasic dispersion at loading up to 140 g L−1 of Mg3AlCO3–LDH in ethyl benzene. At a maximum loading of 160 g L−1 Mg3AlCO3–LDH this mixture becomes a thick monophasic gel.
Introduction
Conventional layered double hydroxides (LDHs) are a family of compounds containing brucite-like layers with general chemical composition [M1−xM′x(OH)2]a+(An−)a/n·bH2O, where M is typically a metal divalent and M′ is typically a trivalent cation, An− is an n-valent anion.1 LDHs have captured much attention in recent years due to their impact across a range of applications such as catalysis,2,3 optics,4 medical science,5,6 and in inorganic–organic nanocomposites.7–10However, LDHs synthesised by conventional methods are often highly aggregated due to their high charge density and hydrophobicity. As a result, isolated LDH powders exhibit relatively low surface areas and unmodified forms cannot be dispersed in non-polar liquids, this imposes severe limitations on their ability to disperse in non-polar liquids or polymers and their ability to be surface functionalised.
Recently, we reported a simple novel method, called the Aqueous Miscible Organic Solvent Treatment (AMOST) method, to obtain a new generation of so called “AMO–LDHs”. For example, AMO–Mg3Al–CO3, AMO–Zn2Al–borate and AMO–Mg3Al–borate LDHs are highly dispersible in non-polar hydrocarbon solvents and exhibit high specific surface area (up to 458.6 m2 g−1).11 In this method, the LDHs are synthesised by conventional methods, e.g. co-precipitation, but the final wet LDH particle suspension is washed and then dispersed in a 100% aqueous miscible organic (AMO) solvent. In some cases AMO solvent treatment of the LDH can lead to dispersion into thin nanosheets or exfoliation to even single layers.12–14 Recent studies have shown that AMO–LDHs have a unique chemical composition given by [M1−xM′x(OH)2]a+(An−)a/n·bH2O·c(AMO-solvent), which instantly distinguishes them from conventional LDHs.15 To date, we have focused on the use of acetone and methanol as the AMO solvents. Here we report a more in depth study of the effect of other AMO solvent treatments on the surface properties of the LDH and their ability to form stable dispersion in range of organic solvents.
Experimental details
Synthesis of Mg3AlCO3–LDH using different organic washing solvents
Mg3AlCO3–LDH was synthesised using a method adapted from the literature.11,15a Mg(NO3)2·6H2O (9.60 g, 37.5 mmol) and Al(NO3)3·9H2O (4.68 g, 12.5 mmol) were dissolved in 50 mL distilled water (Solution A). A second solution was made using Na2CO3 (2.65 g, 25.0 mmol) dissolved in 50 mL distilled water and made to pH 10 by the addition of approximately 5 mL of 1 M HNO3 (Solution B). Solution A was added to Solution B dropwise over 30 minutes with stirring with the pH maintained at pH 10 using 4 M NaOH. The resulting solution was aged with stirring for 16 hours. After aging, the LDH slurry was washed with distilled water at 70 °C until the pH of the washings was pH 7. The slurry was then washed with 200 mL of one of the 12 aqueous miscible organic (AMO) solvents {the solvents used were; 1-propanol, 2-propanol, acetone, acetonitrile, dimethylformamide (DMF), dimethylsulfoxide (DMSO), dioxane, ethanol, ethyl acetate (EA), ethylene glycol (EG), methanol and tetrahydrofuran (THF)} and then dispersed in 200 mL of this solvent for one hour. This washing and dispersion process was repeated on the slurry three times. After washing, the slurry was dried for 24 hours in a vacuum oven.Preparation of dispersions
0.1 g of LDHs was ground into a fine powder using a pestle and mortar, added to 5 mL of the chosen solvent in a glass vial and sonicated for five minutes. The ultrasonic apparatus (CAT no. H100U) was supplied by Essex Scientific Laboratory Supplies Ltd. The samples were allowed to stand for 10 s before the images were taken.Results and discussion
Characterisation of AMO–Mg3AlCO3–LDHs
Mg3AlCO3–LDH was synthesised by co-precipitation method. Prior to drying, samples were treated with twelve different AMO solvents. For comparison, a conventional water-washed Mg3AlCO3–LDH sample was also prepared. The powder X-ray diffraction patterns for the thirteen different samples are displayed in Fig. 1 and S1–S8.†Fig. 1 demonstrates that basic LDH structure is unchanged when the washing solvent is changed from water to an aqueous-miscible solvent. The d-spacing of the 003 Bragg reflection remains ca. 7.9 Å and d-spacing of the 110 Bragg reflection is unchanged from the expected value of 1.5 Å.16 These data indicate that there is no detectible intercalation or swelling of the LDH on AMO-solvent treatment. However, the Bragg reflection peak widths indicate that the crystallinity of the Mg3AlCO3–LDH is affected when the washing solvent is changed from water to a aqueous-miscible solvent.17 The Scherrer equation can be used to provide an estimate of the mean crystallite domain length (CDL).18 An alternative analysis of particle size can be carried out using a whole-pattern fitting method developed by Pielaszek.19 The results of both these analyses are collated in Table 1.
| Washing solvent | CDLa (Å) | CDLb (Å) | Mean sizec (Å) |
|---|---|---|---|
| a Along c-axis, using the 003 Bragg reflection.b In ab-plane, using the 110 Bragg reflection.c Pielaszek method.19 | |||
| Water | 217.0 | 629.4 | 125 |
| 1-Propanol | 145.7 | 654.8 | 80 |
| 2-Propanol | 104.4 | 663.2 | 78 |
| Acetone | 127.3 | 636.4 | 68 |
| Acetonitrile | 121.2 | 651.9 | 71 |
| Dioxane | 138.5 | 669.8 | 71 |
| DMF | 147.0 | 630.0 | 77 |
| DMSO | 126.6 | 652.7 | 77 |
| Ethanol | 135.8 | 631.1 | 74 |
| EA | 136.2 | 645.3 | 71 |
| EG | 130.6 | 639.9 | 69 |
| Methanol | 137.4 | 658.2 | 76 |
| THF | 122.5 | 638.7 | 77 |
These two different methods give different absolute values for the crystallite size; however the crystallinity along the c-axis and the mean particle size show the same overall trend which suggests a reduction in the stacking length of the LDH nanoplates.18 The crystallinity in the ab-plane is largely unchanged with the variation of washing solvent.
Fig. 2 shows the TEM images for a water-washed and an ethanol-washed LDHs.
The darker areas on the TEM image indicate the stacking of the LDH nanosheet crystallites perpendicular to the sample stage. The reduction in these darker areas when the solvent is changed from water to a water-miscible solvent suggests that there is less stacking of the LDH nanosheets, which is consistent with the proposed AMOST mechanism.11
The FTIR spectrum for the ethanol-washed Mg3AlCO3–LDH is displayed in Fig. S9,† it is representative of the IR spectra for all the AMO–LDH synthesised (Fig. S10–S17†). The broad absorption at around 3400 cm−1 is caused by the vibrations and stretching modes associated with –O–H bonds. The absorption at around 1630 cm−1 corresponds to the bending mode of water, indicating that there is still some water present in the samples, even after treatment with an aqueous-miscible solvent. The absorption at around 1360 cm−1 is due to the asymmetric stretching mode of the intercalated carbonate. The absorptions below 1000 cm−1 are due to M − O vibrational modes.20
Surface area analysis of the AMO–Mg3AlCO3–LDHs was performed using N2 BET. The measured surface areas for the LDHs washed with different AMO-solvents is summarised in Fig. 3 It is clear that a change in the washing solvent leads to a dramatic variation in the surface area of the LDH product, all the AMO–LDHs are significantly increased surface area compare to the conventional water-washed sample. The DMF washed Mg3AlCO3–LDH shows the largest surface area of 376 cm2 g−1, which is 3.4 times greater that a conventional water washed sample.
 | ||
| Fig. 3 Nitrogen BET surface areas for the different solvent-treated AMO–Mg3AlCO3–LDHs. 2% error bars are given. The value for the conventional water-washed LDH is from the literature.11 | ||
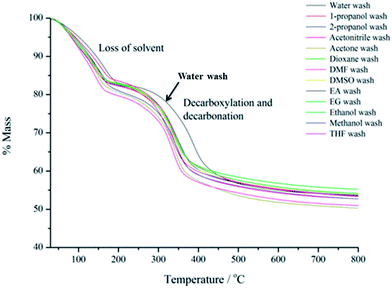
Thermogravimetric analysis (TGA) was used to analyse the thermal decomposition of LDHs. The TGA plots for all samples are displayed in Fig. 4.
The mass loss curves for the AMO Mg3AlCO3–LDHs all share the same characteristic profile expected for the decomposition of an LDH. However, there are some significant differences between the thermal behaviour of water-washed and AMO-solvent washed LDHs in terms of temperature of mass loss and the gradient of the curve. Taking the first derivative of the TGA curves (dTGA) allows changes in mass to be seen more easily, Fig. 5.
The dTGA curve of the water-washed sample is typical of unmodified hydrotalcite.21 The mass loss at ca. 165 °C corresponds to the loss of strongly bound interlayer water. The event at 386 °C corresponds to the almost simultaneous dehydroxylation and decarbonation of the LDH; it is believed that the two peaks overlap. The dTGA curve for the ethanol-washed sample shows two well defined events at low temperature, indicating of the presence of weakly bound solvent. The mass loss of interlayer water takes place at 145 °C and decarboxylation and dehydroxylation take place at around 340 °C. These are both at a lower temperature than the water-washed sample, which indicates that the interlayer solvent and any remaining water molecules are less strongly bound.22 Consistent with the TEM and BET results, the thinner nanosheets and higher surface area of AMO–LDHs also leads to a lower decomposition temperature. Furthermore, we find an additional reproducible feature in the TGA of AMO–LDHs. Different from water-washed sample, there is another small endothermic step between 90–110 °C, which is caused by the ethanol adsorbed on the LDH surface. We find this feature in all AMO–LDHs (see Fig. S18–S26†). This signature enable us do have define a new compositional formula for this family: [M1−xM′x(OH)2]a+(An−)a/n·bH2O·c(AMO-solvent). The details of the composition determined for each AMO–LDH and water-washed LDH are listed in Table S2.†
Organic solvent dispersions of AMO–Mg3AlCO3–LDHs
We have previously reported that acetone treated Mg3AlCO3–LDH may be dispersed in xylenes. We were interested to determine if the different AMO solvent treatment of Mg3AlCO3–LDH would affect their ability to disperse in other liquid solvents. The twelve different AMO solvent treated AMO–Mg3AlCO3–LDHs were then dispersed in eleven different solvents. The aromatic solvents chosen were o-xylene, p-xylene, toluene, tetrahydrofuran (THF), dimethylformamide (DMF) and ethyl benzene in addition to several other non-aromatic solvents 1-methyl-2-pyrrolidone, dichloromethane (DCM), dimethylsulfoxide (DMSO), chloroform and ethanol.In each case a 20 g L−1 loading of the AMO–Mg3AlCO3–LDHs were prepared. The dispersions fell into two classes: opaque and transparent. An example of each is depicted in Fig. 6 which shows a 20 g L−1 acetone-washed Mg3AlCO3–LDHs dispersed in o-xylene, Fig. 6(A) and the equivalent amount dispersed in ethanol, Fig. 6(B).
The only dispersions which were optically transparent were AMO–MgAlCO3–LDHs dispersed in the aromatic solvents; ethyl benzene, o-xylene, p-xylene and toluene, each of these dispersions exhibited the Tyndall effect.23 On standing at room temperature these dispersions were observed to settle into two distinct layers, with the upper layer showing a very weak Tyndall effect and the lower layer showing a high degree of light scattering.
The time taken for the dispersions to settle varied between each AMO-treated LDH from a few seconds to 24 hours. The behaviour of selected dispersions are summarised in Table 2. Full observations are recorded in Table S1.† For some samples, the lower layer was translucent and pearlescent in appearance whereas for others the lower layer was opaque. An example of each is shown in Fig. 7. Neither type of dispersion is stable at this loading and so do not stay as single transparent phase.
| Washing solvent | Dispersion solvent | Time for separation | Lower layer description |
|---|---|---|---|
| 1-Propanol | Ethyl benzene | 6 hours | Very clear, only slightly less transparent than upper layer |
| 2-Propanol | Toluene | 20 seconds | White, solid-like, making up more than half the sample |
| Acetone | o-Xylene | 2 hours | Translucent, pearlescent |
| Acetonitrile | p-Xylene | 24 hours | Translucent, pearlescent |
| Ethylene glycol | o-Xylene | 1 minute | Thick layer of white solid |
| Methanol | p-Xylene | 24 hours | Translucent, almost opaque |
 | ||
| Fig. 7 (A) Images of THF-washed MgAlCO3–LDH dispersed at 20 g L−1 in toluene, (B) washed MgAlCO3–LDH dispersed at 20 g L−1 in toluene. | ||
The results indicate that there is complex phase behaviour for these LDH dispersions. One would expect the dispersions to follow the normal phase behaviour for a regular solution, where at a single temperature the system may be mono- or biphasic (with a solid and a liquid phase) depending on the mole fraction of the solvent. For some two phase systems, there is a point where both phases are truly liquid rather than being a solid phase and a liquid phase. Dispersions similar to Fig. 7(b) appear to be made up of an upper liquid layer and a lower colloidal layer.
A single dispersion with a transparent lower phase was selected and monitored over the course of one week. Fig. 8 shows acetone-washed Mg3AlCO3–LDH dispersed in o-xylene after initial dispersion and after one week. Additional photographs are provided in Fig. S27.†
 | ||
| Fig. 8 Images of acetone-washed Mg3AlCO3–LDH dispersed at 20 g L−1 in o-xylene, (A) initial dispersion and (B) after 1 week. | ||
After settling from the initial single translucent phase into a clear, predominately solvent phase and a lower colloidal phase, the separation remains stable, with no visible LDH crystallites. This may indicate that a true liquid–liquid phase separation has been achieved. Different AMO washing and dispersing solvent combinations lead to different phase behaviour, indicating that there are interactions between the two different solvents and the solid LDH which influence the clarity and stability of the dispersion.
The dispersions which appeared clear after initial addition of LDH display the Tyndall effect were then investigated using UV-vis spectroscopy. The clarity or turbidity was determined by measuring percentage transmittance (T%) between 800 and 200 nm. To aid comparison, T% of the dispersions at 500 nm was recorded.8 Three measurements were taken; the dispersion immediately after agitation, and the upper and lower layers once the dispersion had settled. Results for dispersions in ethyl benzene and o-xylene are summarised in Fig. 9 and 10. Results for dispersions in p-xylene and toluene are displayed in Fig. S28 and S29† respectively.
 | ||
| Fig. 9 Transmission factor (%) at 500 nm of ethyl benzene dispersions at 20 g L−1 for AMO–Mg3AlCO3 LDH treated with a range of organic solvents. | ||
 | ||
| Fig. 10 Transmission factor (%) at 500 nm for o-xylene dispersions at 20 g L−1 for AMO–Mg3AlCO3 LDH treated with a range of organic solvents. | ||
A ‘good’ dispersion was defined as one with high transparency (T% ≥ 50%) in both the initial dispersion and the lower layer of the biphasic system. We can use optical clarity is a measure of how well dispersed LDHs are in each solvent. If the lower phase is not colloidal, it will show very low transparency; the ‘best’ dispersions will be those where the lower layer is most transparent, as this will indicate a high level of dispersion of the LDH. The lower layer was also examined visually to check if any large aggregates of LDH particles formed. For example, 2-propanol-washed LDH produces a good dispersion on addition to ethyl benzene (T% = 85.4% in initial sample and 74.1% in lower layer) and DMSO-washed LDH forms a poor dispersion in o-xylene (T% = 56.3% in initial dispersion and 12.9% in lower layer). For each dispersion solvent, an optimum pairing was selected for further investigation. The pairings were: 1-propanol-washed LDH in ethyl benzene, methanol-washed LDH in o-xylene, methanol-washed LDH in p-xylene and acetonitrile-washed LDH in toluene.
With each of the optimum pairings, an investigation was carried out into the effect of increasing the LDH loading on the biphasic character, transparency and thickness of the dispersions. Fig. 11 and 12 show how T% changed with increasing loading for 1-propanol-washed LDH dispersed in ethyl benzene and acetonitrile-washed LDH dispersed in toluene. Data for dispersions of methanol-washed LDH in o- and p-xylene are displayed in Fig. S30 and S31† respectively.
 | ||
| Fig. 11 The variation in transmission factor (%) at 500 nm of increasing loading for 1-propanol-washed Mg3AlCO3 dispersed in ethyl benzene. | ||
 | ||
| Fig. 12 The variation in transmission factor (%) at 500 nm of increasing loading for acetonitrile-washed Mg3AlCO3 LDH dispersed in toluene. | ||
For 1-propanol-washed LDH dispersed in ethyl benzene, Fig. 11, the biphasic character was optically difficult to identify up to a loading of 40 g L−1, but was detected using UV-vis spectroscopy from 10 g L−1 loading (T% upper layer = 99.4%, T% lower layer = 98.0%). At a loading of 140 g L−1, the dispersion became monophasic (ΔT% = 0). Maximum loading was achieved at 160 g L−1, when the sample became a thick, monophasic gel.
For acetonitrile-washed LDH dispersed in toluene, Fig. 12, the dispersion did not display biphasic character until 20 g L−1 loading (T% upper layer = 96.5%, T% lower layer = 76.8%). At a loading of 50 g L−1, it became a single translucent phase and a thick, translucent gel (ΔT% = 0). For methanol-washed LDH in o-xylene, biphasic character was not apparent until 10 g L−1 loading, Fig. S30.† At a loading of 70 g L−1 it became a single translucent phase, and at 90 g L−1 it became a thick translucent gel. For methanol-washed LDH in p-xylene, biphasic character was observed for all loadings up to 60 g L−1, when a single translucent phase was seen, Fig. S31.† The dispersion thickened with loading and became an immobile gel at a loading of 80 L−1.
‘Good’ dispersions were those that showed high transparency in the initial sample and of the lower phase once the sample had settled into two phases. Reasons for favourable dispersion properties are likely to be related to the properties of the solvent used for washing or the interaction between the washing and dispersion solvent. There does not appear to be a correlation between the polarity of the washing solvent and the dispersion solvent for dispersions that showed a high level of transparency. For the ‘best’ pairings that were investigated, the boiling point of the washing solvent was lower than the boiling point of the dispersion solvent. LDHs washed with solvents with boiling points higher than the dispersion solvent dispersed poorly (e.g. DMF and DMSO).
Properties of the LDH obtained after washing (e.g. a change in surface area) may also be a factor in how well the LDH disperses. However, the LDHs which gave the most transparent dispersions display very different surface areas; 1-propanol-washed Mg3AlCO3–LDH had the smallest surface area at 177 cm−1, whilst acetonitrile-washed Mg3AlCO3–LDH exhibited a very large surface area of 284 cm−1.
Conclusions
Aqueous Miscible Organic Solvent Treated (AMOST) Mg3AlCO3–LDHs have been prepared using twelve different organic treatment solvents. The AMOST process produces significant changes to the crystallinity, morphology, thermal behaviour and dramatic increases the surface areas of the LDHs.AMOST-modified Mg3AlCO3–LDH can form clear dispersions in a range of aromatic solvents at high loadings. To date, our experiments concluded that the optimal dispersion is a 1-propanol treated Mg3AlCO3–LDHs dispersed in ethyl benzene. This system produces a monophasic dispersion at loading up to 140 g L−1 in ethyl benzene. At a maximum loading of 160 g L−1, this mixture becomes a thick monophasic gel.
Acknowledgements
M.Y. and J.-C.B. would like to thank SCG Chemicals Ltd, Thailand for funding.Notes and references
- (a) X. Duan and D. G. Evans, Layered double hydroxides, Springer Verlag, 2006 CrossRef; (b) V. Rives, Layered double hydroxides: present and future, Nova Science Publishers, 2001 Search PubMed; (c) F. Cavani, F. Trifiro and A. Vaccari, Catal. Today, 1991, 11, 173 CrossRef CAS.
- X. Zou, A. Goswami and T. Asefa, J. Am. Chem. Soc., 2013, 135, 17242 CrossRef CAS PubMed.
- Y. Zhao, B. Li, Q. Wang, W. Gao, C. J. Wang, L. Zheng, M. Wei, D. G. Evans, X. Duan and D. O'Hare, Chem. Sci., 2014, 5, 951 RSC.
- Z. Liu, R. Ma, M. Osada, N. Iyi, Y. Ebina, K. Takada and T. Sasaki, J. Am. Chem. Soc., 2006, 128, 4872 CrossRef CAS PubMed.
- X. Gao, L. Lei, D. O'Hare, J. Xie, P. Gao and T. Chang, J. Solid State Chem., 2013, 203, 174 CrossRef CAS PubMed.
- J.-H. Choy, S.-Y. Kwak, Y.-J. Jeong and J.-S. Park, Angew. Chem., Int. Ed., 2000, 39, 4042 CrossRef CAS.
- (a) Q. Wang, J. Undrell, Y. Gao, G. Cai, J.-C. Buffet, C. A. Wilkie and D. O'Hare, Macromolecules, 2013, 46, 6145 CrossRef CAS; (b) Y. Gao, J. Wu, Q. Wang, C. A. Wilkie and D. O'Hare, J. Mater. Chem. A, 2014, 2, 10996 RSC.
- Q. Wang, X. Zhang, J. Zhu, Z. Guo and D. O'Hare, Chem. Commun., 2012, 48, 7450 RSC.
- S. Abedi and M. Abdouss, Appl. Catal., A, 2014, 475, 386 CrossRef CAS PubMed.
- (a) Y. Gao, Z. Zhang, J. Wu, X. Yi, A. Zheng, A. Umar, D. O'Hare and Q. Wang, RSC Adv., 2013, 3, 26017 RSC; (b) F.-A. He and L.-M. Zhang, Compos. Sci. Technol., 2007, 67, 3226 CrossRef CAS PubMed; (c) F.-A. He and L.-M. Zhang, J. Colloid Interface Sci., 2007, 315, 439 CrossRef CAS PubMed.
- Q. Wang and D. O'Hare, Chem. Commun., 2013, 49, 6301 RSC.
- K. Li, G. Wang, D. Li, Y. Lin and X. Duan, Chin. J. Chem. Eng., 2013, 21, 453 CrossRef CAS.
- (a) J. Oh, S. Hwang and J. Choy, Solid State Ionics, 2002, 151, 285 CrossRef CAS; (b) A. Alvarez, R. Trujillano and V. Rives, Appl. Clay Sci., 2013, 80–81, 326 CrossRef CAS PubMed.
- (a) S. Zeng, X. Xu, S. Wang, Q. Gong, R. Liu and Y. Yu, Mater. Chem. Phys., 2013, 140, 159 CrossRef CAS PubMed; (b) Z. Huang, P. Wu, B. Gong, Y. Fang and N. Zhu, J. Mater. Chem. A, 2014, 2, 5534 RSC; (c) L. Cchen, K. Sun, P. Li, X. Fan, J. Sun and S. Ai, Nanoscale, 2013, 5, 10982 RSC; (d) H. Chen, L. Hu, M. Chen, Y. Yan and L. Wu, Adv. Funct. Mater., 2014, 24, 934 CrossRef CAS; (e) Z. Hu and G. Chen, RSC Adv., 2013, 3, 12021 RSC; (f) Z. P. Xu, G. Stevenson, C.-Q. Lu and G. Q. M. Lu, J. Phys. Chem. B, 2006, 110, 16923 CrossRef CAS PubMed.
- (a) C. Chen, M. Yang, Q. Wang, J.-C. Buffet and D. O'Hare, J. Mater. Chem. A, 2014, 2, 15102–15110 RSC; (b) N. P. Funnell, Q. Wang, L. Connor, M. G. Tucker, D. O'Hare and A. L. Goodwin, Nanoscale, 2014, 6, 8032 RSC; (c) Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124 CrossRef CAS PubMed.
- (a) Q. Tao, H. He, R. L. Frost, P. Yuan and J. Zhu, J. Therm. Anal. Calorim., 2009, 101, 153 CrossRef PubMed; (b) B. Kutlu, A. Leuteritz, R. Boldt, D. Jehnichen and G. Heinrich, Chem. Eng. J., 2014, 243, 394 CrossRef CAS PubMed.
- G. Hu, N. Wang, D. O'Hare and J. Davis, J. Mater. Chem., 2007, 17, 2257 RSC.
- (a) R. E. Dinneier and S. J. L. Billinge, S. J. L. Principles of Powder Diffraction, in Powder Diffraction: Theory and Practice, ed. R. E. Dinneier and S. J. L. Billinge, Royal Society of Chemistry, 2008, pp. 1–19 Search PubMed; (b) J. I. Langford and A. J. C. Wilson, J. Appl. Crystallogr., 1978, 11, 102 CrossRef CAS; (c) Q. Wang, X. Zhang, C. J. Wang, J. Zhu, Z. Guo and D. O'Hare, J. Mater. Chem., 2012, 22, 19113 RSC.
- (a) R. Pielaszek, J. Alloys Compd., 2004, 382, 128 CrossRef CAS PubMed; (b) T. Wejrzanowski, R. Pielaszek, A. Opalińska, H. Matysiak, W. Łojkowski and K. J. Kurzydłowski, Appl. Surf. Sci., 2006, 253, 204 CrossRef CAS PubMed.
- (a) Q. Tao, J. Zhu, R. L. Frost, T. E. Bostrom, R. M. Wellard, J. Wei, P. Yuan and H. He, Langmuir, 2010, 26, 2769 CrossRef CAS PubMed; (b) Q. Tao, J. Zhu, R. M. Wellard, T. E. Bostrom, R. L. Frost, P. Yuan and H. He, J. Mater. Chem., 2011, 21, 10711 RSC; (c) A. de Roy, C. Forano, and J. P. Besse, Layered Double Hydroxides: Synthesis and Post-Synthesis Modification, in Layered Double Hydroxides: Present and Future, ed. V. Rives, Nova Publishers, 2001, pp. 39–50 Search PubMed.
- F. L. Theiss, G. A. Ayoko and R. L. Frost, J. Therm. Anal. Calorim., 2012, 112, 649 CrossRef.
- (a) S. Palmer, R. Frost and T. Nguyen, Coord. Chem. Rev., 2009, 253, 250 CrossRef CAS PubMed; (b) F. Malherbe and J.-P. Besse, J. Solid State Chem., 2000, 155, 332 CrossRef CAS.
- D. Ebbing and S. D. Gammon, General Chemistry, Enhanced Edition, Cengage Learning, 2010, p. 509 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterisations. See DOI: 10.1039/c4ra08505a |
| This journal is © The Royal Society of Chemistry 2014 |