Experimental evaluation of the gas recovery factor of methane hydrate in sandy sediment
Abstract
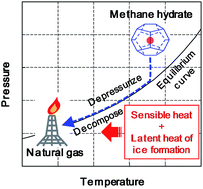
Gas production tests have been conducted on artificial sandy sediments saturated by methane hydrate and water using a unique apparatus referred to as High-pressure Giant Unit for Methane-hydrate Analyses (HiGUMA), which is the world's largest reservoir simulating vessel intended for gas hydrate analysis. The gas recovery factor was investigated at various depressurization schemes, including one-step depressurization, multistep depressurization, and depressurization below the quadruple point of methane hydrate. The gas production rate increased during the depressurization process with sediment temperature reduction; however, the rate decrease and stabilized at a very low level after the temperature reached a newly established equilibrium condition. This result indicates that an appropriate heat of the hydrate-bearing sediments is a crucial factor for driving hydrate dissociation. The potential economic recovery factor was 14% for 4.6 MPa of production pressure in the one-step depressurization. In the multistep depressurization, the recovery factor was increased with a reduction in production pressure and showed values of 13%, 31%, and 40% for 4.0 MPa, 3.1 MPa, and 2.5 MPa, respectively. However, depressurization above the quadruple point could not dissociate all the existing hydrate due to the lack of heat. In contrast, it was determined that 65% of the in-place methane could be produced when the production pressure was decreased to 2.1 MPa, which is below the quadruple point, because the latent heat of ice formation was efficiently used for hydrate dissociation. The results show that intentional ice formation by adjusting production pressure can potentially enhance methane hydrate recovery at a comparable level of conventional natural gas production.

 Please wait while we load your content...
Please wait while we load your content...