Synthesis of oxazepin-quinoxaline bis-heterocyclic scaffolds via an efficient three component synthetic protocol†
Fatemeh Hajishaabanha and
Ahmad Shaabani*
Faculty of Chemistry, Shahid Beheshti University, G. C., P. O. Box 19396-4716, Tehran, Iran. E-mail: a-shaabani@sbu.ac.ir
First published on 15th September 2014
Abstract
An efficient protocol has been developed to access a series of novel highly complex bis-heterocyclic oxazepin-quinoxaline derivatives. The new bifunctional compounds were synthesized via a one-pot three component reaction between 2-(2-formylphenoxy)acetic acid, an amine and 6-hydroxybenzo[f]quinoxaline-2,3-dicarbonitrile in toluene under reflux conditions without using any catalyst in good to excellent yields.
Oxazepines as a “privileged scaffold” are a well-known class of seven-membered heterocycles with two heteroatoms. The molecular properties of this pharmaceutically important nucleus have been extensively studied due to its presence in some natural products and biologically active compounds.1 Among the biological activities, it is worth mentioning antithrombotic,2 antiepileptic,3 anticonvulsant,4 anti-inflammatory,5 progesterone agonist,6 antifungal,7 antagonist and analgesic,8 antipsychotic,9 anxiolytics,10 antihistaminic,11 antiaggregating,12 and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitory13 activities. Moreover, among compounds containing this fragment, Sintamils (I)14 and its derivatives as antidepressants and Ioxapine15 because of its potential clozapine-like properties were reported (Fig. 1).16
As a result of the above-mentioned properties of oxazepine frameworks, a variety of methods have been introduced for the preparation of these compounds.17 For example, a synthesis via a copper-catalyzed cascade reaction has been reported by Nakamura.18 Reiser and co-workers achieved combinatorial liquid-phase synthesis of [1,4]oxazepine-7-ones via the Baylis–Hillman reaction.19 Menendez and co-workers reported syntheses of oxazepine scaffolds through a CAN-catalyzed four-component reaction.20 In addition, the use of a novel modification of four-component Ugi condensation for synthesis of oxazepines has been reported by Ivachtchenko.21
Nowadays, the combination of several functional groups in one molecule seems as a useful tool to optimize properties of biological compounds. Quinoxaline frameworks have attracted considerable attention due to their presence in a large variety of physiologically active compounds, with applications varying from medicinal to agricultural.22 Besides, this chemical-building block has been used to generate different materials to be used in the field of new technologies.23 Accordingly, the incorporation of quinoxalines into oxazepines may potentially provide a class of novel drug candidates with unusual biological activities.
Inspired by the above investigation and our continued interest in the development of new synthetic methods for generation of heterocyclic compounds,24 herein we wish to report synthesis of oxazepin-quinoxaline bis-heterocyclic scaffold 4 by a one-pot three-component condensation reaction of 2-(2-formylphenoxy)acetic acid 1 as a bifunctional building block,25 amines 2 as a N-resource and 6-hydroxybenzo[f]quinoxaline-2,3-dicarbonitrile 3 as a nucleophilic reagent in toluene under reflux conditions without using any catalyst (Table 2).
| Entry | Solvent | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 2-(2-formylphenoxy)acetic acid 1 (1 mmol), benzyl amine 2a (1 mmol), 6-hydroxybenzo[f]quinoxaline-2,3-dicarbonitrile 3 (1 mmol), solvent (5 mL).b Isolated yields.c NR (no reaction). | ||||
| 1 | MeOH | 64 °C | 24 | Trace |
| 2 | EtOH | 78 °C | 24 | Trace |
| 3 | CH3CN | 81 °C | 24 | 80 |
| 4 | H2O | 100 °C | 24 | NR |
| 5 | AcOH | 118 °C | 24 | Trace |
| 6 | Toluene | 110 °C | 24 | 96 |
| 7 | H2O/AcOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
100 °C | 24 | 10 |
| 8 | EtOH/AcOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
78 °C | 24 | 15 |
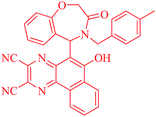
In a pilot experiment, 2-(2-formylphenoxy)acetic acid 1, benzyl amine 2a and 6-hydroxybenzo[f]quinoxaline-2,3-dicarbonitrile 3, which was prepared according to our previously reported method,26 were refluxed in toluene. The progress of reaction was monitored by TLC. After 24 h, the reaction was completed and 5-(4-benzyl-3-oxo-2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-5-yl)-6-hydroxybenzo[f]quinoxaline-2,3-dicarbonitrile 4a was obtained in 96% yield (Scheme 1).
In order to obtain the best media, the reaction was investigated in various solvents of different polarity and viscosity. As indicated in Table 1, the efficiency and the yield of the reaction in toluene were higher than those obtained in other solvents.
With the optimized reaction conditions in hand, we began to evaluate the substrate scope of structurally various amines. As indicated in Table 2, the one-pot three component reactions proceeded very efficiently under reflux conditions to produce the corresponding 2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-5-ylbenzo[f]quinoxaline-2,3-dicarbonitrile derivatives 4a–h in good to excellent yields.
The structures of compounds 4a–h were deduced from their IR, mass, 1H NMR, and 13C NMR spectral data. The spectroscopic data of desired products were in accordance with the assigned structures. For example, the 1H NMR spectrum of 4a exhibited two ABq for CH2 of benzyl and OCH2 at δ = 4.25 and 4.80, a singlet at δ = 6.43 for CH and a multiplet and two doublet at δ = 6.78–8.92 for H-aromatic and OH. The 1H-decoupled 13C NMR spectrum of 4a showed 28 distinct resonances in agreement with the proposed structure. The mass spectra of these compounds displayed molecular ion peaks at the appropriate m/z values.
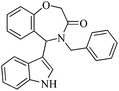
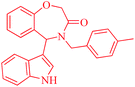
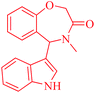
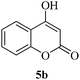
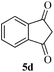
It is worth noting that we also investigated the reaction between different nucleophilic reagents 5, 2-(2-formylphenoxy)acetic acid 1 and benzyl amine 2a under the previous-mentioned optimized conditions. As indicated in Table 3, only the reaction carried out very well with indole 5a affording the desired product 6aa in 85% yield. In the case of entries 6ab–ae, imine formation, did not proceed in the presence of nucleophiles 5b–5e. Presumably, these observations may be due to the low nucleophilicity of these nucleophiles in comparison with indole. In view of the success of the mentioned reaction, we explored the scope and limitations of this reaction, by extending the procedure to various amines 2, 2-(2-formylphenoxy)acetic acid 1 and indole 5a. As shown in Table 3, the products 6aa, 6ba, 6da and 6fa were produced in 72–85% yields and the structures of them were identified by its IR and 1H NMR and mass spectral data.
On the basis of experimental results, a suggested mechanism for the formation of 2,3,4,5-tetrahydrobenzo[f][1,4]oxazepin-5-ylbenzo[f]quinoxaline-2,3-dicarbonitrile derivatives 4a–h is presented in Scheme 2. It is conceivable that the initial event in this reaction is the nucleophilic attack of amines 2 to formyl group to afford the imine intermediate 7,25a,b which subsequently reacts with 3 to form intermediate 8. Finally, intermediate 8 undergoes an intramolecular cyclization25a,b to give the final product 4.
Conclusions
In summary, we have successfully demonstrated a novel and highly efficient one-pot strategy for the synthesis of complex bis-heterocyclic oxazepin-quinoxalines which are two important pharmacological and biological scaffolds through three component reactions of 6-hydroxybenzo[f]quinoxaline-2,3-dicarbonitrile, 2-(2-formylphenoxy)acetic acid and structurally diverse amines in good to excellent isolated yields. Moreover, it is worth mentioning that this operationally simple, practical, and scalable method allows C–C bond and C–N bond formation with excellent scope. The potential uses of this protocol in synthetic and medicinal chemistry may be remarkable, since the products share structural and functional group properties of the biologically active molecules.Experimental
General procedure
A mixture of 2-(2-formylphenoxy)acetic acid 1 (0.18 g, 1 mmol), amines 2 (1 mmol), and 6-hydroxybenzo[f]quinoxaline-2,3-dicarbonitrile 3 (0.25 g, 1 mmol) in toluene (5 mL) was refluxed for 24 h. After completion of the reaction, as indicated by TLC (ethyl acetate/n-hexane, 3/1), the precipitate was filtered and washed with toluene. The products were further purified by recrystallization in acetone.Acknowledgements
We gratefully acknowledge financial support from the Iran National Science Foundation (INSF) and Research Council of Shahid Beheshti University.Notes and references
- (a) C. Ma, S.-J. Liu, L. Xin, J. R. Falck and D.-S. Shin, Tetrahedron, 2006, 62, 9002 CrossRef CAS PubMed; (b) M. H. Serrano-Wu, D. R. S. Laurent and Y. Chen, Bioorg. Med. Chem. Lett., 2002, 12, 2757 CrossRef CAS; (c) P. Matyus, I. Varga, E. Zara, A. Mezei, A. Behr and A. Simay, Bioorg. Med. Chem. Lett., 1997, 7, 2857 CrossRef CAS; (d) E. A. Hallinan, T. J. Hagen, S. Tsymbalov, A. Stapelfeld and M. A. Savage, Bioorg. Med. Chem., 2001, 9, 1 CrossRef CAS; (e) P. P. M. A. Dols, B. J. B. Folmer, H. Hamersma, C. W. Kuil, H. Lucas, L. Ollero, J. B. M. Rewinkel and P. H. H. Hermkens, Bioorg. Med. Chem. Lett., 2008, 18, 1461 CrossRef CAS PubMed; (f) O. Hasnah, M. AbdulKarim-Talaq, Y. Guan-Yeow and A. Farook, Chin. J. Chem., 2011, 29, 1518 CrossRef; (g) K. Audouze, E. Q. Nielsen and D. Peters, J. Med. Chem., 2004, 47, 3089 CrossRef CAS PubMed; (h) I. Ott, B. Kircher, G. Heinisch and B. Matuszczak, J. Med. Chem., 2004, 47, 4627 CrossRef CAS PubMed.
- (a) J. K. Mishra, K. Samanta, M. Jain, M. Dikshit and G. Panda, Bioorg. Med. Chem. Lett., 2010, 20, 244 CrossRef CAS PubMed; (b) H. Agirbas, B. Kemal and F. Budak, Med. Chem. Res., 2011, 20, 1170 CrossRef CAS.
- A. Pekcec, B. Unkruer, J. Schlichtigeret, J. Soerensen, A. M. S. Hartz, B. Bauer, E. A. van Vliet, J. A. Gorter and H. Potschka, J. Pharmacol. Exp. Ther., 2009, 330, 939 CrossRef CAS PubMed.
- G. Sharma, J. Y. Park and M. S. Park, Arch. Pharmacal Res., 2008, 31, 838 CrossRef CAS PubMed.
- (a) D. R. Schridhar, C. R. Sarma and R. R. Krishna, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1979, 17B, 155 Search PubMed; (b) N. K. Verma, E. Dempsey, J. Conroy, P. Olwell, A. M. Mcelligott, A. M. Davies, D. Kelleher, S. Butini, G. Campiani, D. C. Williams, D. M. Zisterer, M. Lawler and Y. Volkov, J. Mol. Med., 2008, 86, 457 CrossRef CAS PubMed.
- P. P. M. A. Dols, B. J. B. Folmer, H. Hamersma, C. W. Kuil, H. Lucas, L. Ollero, J. B. M. Rewinkel and P. H. H. Hermkens, Bioorg. Med. Chem. Lett., 2008, 18, 1461 CrossRef CAS PubMed.
- M. H. Serrano-Wu, D. R. St Laurent, Y. J. Chen, S. Huang, K.-R. Lam, J. A. Matson, C. E. Mazzucco, T. M. Stickle, T. P. Tully, H. S. Wong, D. M. Vyas and B. N. Balasubramanian, Bioorg. Med. Chem. Lett., 2002, 12, 2757 CrossRef CAS.
- (a) F. Okada, Y. Torii, H. Saito and N. Matsuki, Jpn. J. Pharmacol., 1994, 64, 109 CrossRef CAS; (b) E. A. Hallinan, A. Stapelfeld, M. A. Savage and M. Reichman, Bioorg. Med. Chem. Lett., 1994, 4, 509 CrossRef CAS.
- J. F. F. Liegeois, F. A. Rogister, J. Bruhwyler, J. Damas, T. P. Nguyen, M. O. Inarejos, E. M. G. Chleide, M. G. A. Mercier and J. E. Delarge, J. Med. Chem., 1994, 37, 519 CrossRef CAS.
- R. C. Effland, G. C. Helsley and J. J. Tegeler, J. Heterocycl. Chem., 1982, 19, 537 CrossRef CAS.
- M. C. Sleevi, A. D. Cale Jr, T. W. Gero, L. W. Jaques, W. J. Welstead, A. F. Johnson, B. F. Kilpatrick, I. Demian, J. C. Nolan and H. Jenkins, J. Med. Chem., 1991, 34, 1314 CrossRef CAS.
- J. Aono, M. Sugawa, T. Koide and M. Takato, Eur. J. Pharmacol., 1991, 195, 225 CrossRef CAS.
- L. Smith II, W. C. Wong, A. S. Kiselyov, S. Burdzovic-Wizemann, Y. Mao, Y. Xu, M. A. J. Duncton, K. Kim, E. L. Piatnitski, J. F. Doody, Y. Wang, R. L. Rosler, D. Milligan, J. Columbus, C. Balagtas, S. Ping Lee, A. Konovalov and Y. R. Hadari, Bioorg. Med. Chem. Lett., 2006, 16, 5102 CrossRef PubMed.
- K. Nagarajan, J. David, Y. S. Kulkarni, S. B. Hendi, S. J. Shenoy and P. Upadhyaya, Eur. J. Med. Chem., 1986, 21, 21 CAS.
- Y. Liao, B. J. Venhuis, N. Rodenhuis, W. Timmerman and H. Wikstrom, J. Med. Chem., 1999, 42, 2235 CrossRef CAS PubMed.
- (a) A. V. Samet, V. N. Marshalkin, K. A. Kislyi, N. B. Chernysheva, Y. A. Strelenko and V. V. Semenov, J. Org. Chem., 2005, 70, 9371 CrossRef CAS PubMed; (b) Y. Liu, C. Chu, A. Huang, C. Zhan, Y. Ma and C. Ma, ACS Comb. Sci., 2011, 13, 547–553 CrossRef CAS PubMed.
- (a) R. Narquizian, T. Woltering and W. Wostl, US. Pat., 2012, 2012/0253035 A1; (b) M. Ghandi, A. Olyaei and S. Raoufmoghaddam, J. Heterocycl. Chem., 2009, 46, 914 CrossRef CAS; (c) M. Ghandi, T. Momeni, M. T. Nazeri, N. Zarezadeh and M. Kubicki, Tetrahedron Lett., 2013, 54, 2983 CrossRef CAS PubMed; (d) F. Khaleghi, L. Bin Din, I. Jantan, W. A. Yaacob and M. A. Khalilzadeh, Tetrahedron Lett., 2011, 52, 7182 CrossRef CAS PubMed.
- I. Nakamura, Y. Kudo and M. Terada, Angew. Chem., Int. Ed., 2013, 52, 1 CrossRef.
- R. Racker, K. Doring and O. Reiser, J. Org. Chem., 2000, 65, 6932 CrossRef PubMed.
- V. Sridharan, S. Maiti and J. C. Menendez, J. Org. Chem., 2009, 74, 9365 CrossRef CAS PubMed.
- A. P. Ilyin, V. Z. Parchinski, J. N. Peregudova, A. S. Trifilenkov, E. B. Poutsykina, S. E. Tkachenko, D. V. Kravchenko and A. V. Ivachtchenko, Tetrahedron Lett., 2006, 47, 2649 CrossRef CAS PubMed.
- (a) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed; (b) A. E. Porter, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon, Oxford, 1984, vol. 3, Pt. 2B, p. 157 Search PubMed; (c) A. K. Patidar, M. Jeyakandan, A. K. Mobiya and G. Selvam, Int. J. PharmTech Res., 2011, 3, 386 CAS; (d) V. A. Mamedov and N. A. Zhukova, Progress in Quinoxaline Synthesis, in Progress in Heterocyclic Chemistry, Elsevier, 2012, vol. 24, ch. 1, Part 1, p. 55 Search PubMed.
- M. González and H. Cerecetto, Expert Opin. Ther. Pat., 2012, 22, 1289 CrossRef PubMed.
- (a) R. Ghadari, F. Hajishaabanha, M. Mahyari, A. Shaabani and H. R. Khavasi, Tetrahedron Lett., 2012, 53, 4018 CrossRef CAS PubMed; (b) A. Shaabani, F. Hajishaabanha, M. Mahyari, H. Mofakham and S. W. Ng, Tetrahedron, 2011, 67, 8360 CrossRef CAS PubMed; (c) A. Shaabani, A. Maleki, F. Hajishaabanha, H. Mofakham, M. Seyyedhamzeh, M. Mahyari and S. W. Ng, J. Comb. Chem., 2010, 12, 186 CrossRef CAS PubMed; (d) A. Shaabani, A. Maleki, H. Mofakham and J. Moghimi-Rad, J. Org. Chem., 2008, 73, 3925 CrossRef CAS PubMed; (e) A. Shaabani, H. Mofakham, A. Maleki and F. Hajishaabanha, J. Comb. Chem., 2010, 12, 630 CrossRef CAS PubMed; (f) S. Shaabani, A. Shaabani and S. W. Ng, ACS Comb. Sci., 2014, 16, 176 CrossRef CAS PubMed.
- (a) H. You, F. Chen, M. Lei and L. Hu, Tetrahedron Lett., 2013, 54, 2972 CrossRef CAS PubMed; (b) S.-C. Shen, X.-W. Sun and G.-Q. Lin, Green Chem., 2013, 15, 896 RSC; (c) S.-C. Shen, X.-W. Sun and L. Guoqiang, Synthesis, 2013, 45, 1181 CrossRef CAS PubMed; (d) Z. Xu, M. Ayaz, A. A. Cappelli and C. Hulme, ACS Comb. Sci., 2012, 14, 460 CrossRef CAS PubMed; (e) S. Gunawan and C. Hulme, Org. Biomol. Chem., 2013, 11, 6036 RSC; (f) K. Siva Kumar, P. Mahesh Kumar, K. Anil Kumar, M. Sreenivasulu, A. A. Jafar, D. Rambabu, G. Rama Krishna, C. Malla Reddy, R. Kapavarapu, K. Shivakumar, K. Krishna Priya, K. V. L. Parsa and M. Pal, Chem. Commun., 2011, 47, 5010 RSC.
- R. Ghadari, F. Hajishaabanha, M. Aghaei, A. Shaabani and S. W. Ng, Mol. Diversity, 2012, 16, 453 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08486a |
| This journal is © The Royal Society of Chemistry 2014 |