Onset of mixing-induced thixotropy in hydrogels by mixing two homologues of low-molecular-weight hydrogelators†
Yutaka Ohsedo*a,
Masashi Oonob,
Kowichiro Saruhashib and
Hisayuki Watanabeab
aAdvanced Materials Research Laboratory, Collaborative Research Division, Art, Science and Technology Center for Cooperative Research, Kyushu University, 4-1 Kyudaishinmachi Nishi-ku, Fukuoka 819-0388, Japan. E-mail: ohsedo@astec.kyushu-u.ac.jp; Fax: +81-92-400-4382; Tel: +81-92-400-4381
bNissan Chemical Industries, Ltd., 2-10-1 Tsuboinishi, Funabashi, Chiba 274-8507, Japan
First published on 8th September 2014
Abstract
We determined the onset of thixotropy in hydrogels when two homologues of low-molecular-weight hydrogelators, N-alkyl-D-glucamides, were mixed. Intriguingly, the hydrogels generated from the individual components did not show such behaviour. The mixing induced onset of thixotropy might be attributed to the improved network quality.
Hydrogels, which are hydrophilic polymeric materials, facilitate the activity of cells1a and have applications in pharmaceuticals,1b biomedicine,1c,d and tissue engineering.1e,f Creation of novel hydrogels that are different from ordinary hydrogels, which are primarily composed of polymers, is a developing field in materials chemistry. Low-molecular-weight gelators (LMWGs)2,3 are novel hydrogels, which show unique properties comparable to polymer-based hydrogels, such as multi-stimuli responsiveness,4 and have potential applications in medicinal and optoelectronic fields.5,6 For creating such types of LMWGs extensive investigation is required for suitable molecular motifs containing various functionalities. Moreover, methods to easily and effectively add functionalities to hydrogels made from known LMWGs need to be explored.
Here, we report the functionalization of hydrogels by mixing two LMWGs, N-alkyl-D-glucamides, with alkyl chains of different lengths. We observed that mixing of the two components enhances the gelation effect and mechanical properties of the two-component systems, especially the thixotropic property, which is known as shear thinning,7 and other unique properties related to molecular network in the hydrogel.8 Previously, we reported the enhancement in thixotropic properties of organogels by mixing LMWGs (alkylhydrazides, alkylamides or alkylureas having different alkyl chains).9 We now extend this two-component mixing method for the creation of a new hydrogel based on N-alkyl-D-glucamide, which is a LWMG that has been studied extensively by Fuhrhop et al. and other researchers.10–14 Note that hydrogelation properties and the microstructure of crystal fibres formed by mixing the two N-alkyl-D-glucamides (N-octyl and N-dodecyl) have also been investigated;10c however, mixing induced onset of thixotropy is the focus of this report. Several systems that involve mixing-induced enhancement of gel properties or formation of gels have been extensively investigated.15,16 Our strategy involves simply mixing homologues of LMWGs; these LMWG homologues contain the same polar hydrogen bonding moiety with hydrophobic alkyl chains of different lengths.
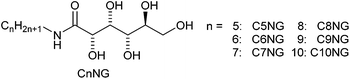
The synthesis of N-alkyl-D-glucamides, CnNG (n = 5–10) with alkyl chains of different lengths was performed following established procedures (Scheme 1).10a,b Initially, we evaluated the hydrogelation ability of each compound. Table 1 lists the critical gelation concentration (CGC) for each CnNG, i.e. one-component hydrogels. As described previously in literature,10c C8NG formed an opaque hydrogel, but started to crystallize within several days. Furthermore, C10NG, C9NG and C7NG formed opaque hydrogels, but crystallized within several hours, whereas C5NG remained as an aqueous solution after the gelation test. A weak hydrogelation was observed with C6NG (7 wt%), but no crystallization was observed over a 3 month period. Next, we evaluated hydrogelation with binary mixtures of CnNGs (Table 1). In these results, all hydrogels, i.e. two-component hydrogels, were either opaque or turbid, and the hydrogelation ability of different CnNG mixtures showed gelation ability that is either similar to or better than individual components. The poor hydrogelation ability of C7NG/C6NG (5 wt%) was probably due to the high CGC of C6NG and the tendency of C7NG to appropriate CnNGs enhanced the hydrogelation abilities of the mixture and inhibited the tendency of the hydrogelators to crystallize.
| Sample | CGC (wt%) | Sample ratioa (w/w) | CGC (wt%) | Sample ratioa (w/w) | CGC (wt%) |
|---|---|---|---|---|---|
| a ‘Cn’, etc. denotes CnNG. wt% refers to the total weight of all of the components. Key: K: solidified with crystalline appearance. S: solution at the concentration. PG: partial gel at the concentration. | |||||
| C10NG | 5.0 | C10/C9 1/1 | 5.0 (PG) | C9/C7 1/1 | 3.0 |
| C9NG | 3.0 | C10/C8 1/1 | 3.0 | C9/C6 1/1 | 3.0 |
| C8NG | 3.0 | C10/C7 1/1 | 3.0 | C8/C7 1/1 | 2.0 |
| C7NG | 3.0 (K) | C10/C6 1/1 | 3.0 | C8/C6 1/1 | 2.0 |
| C6NG | 7.0 | C9/C8 1/1 | 3.0 | C7/C6 1/1 | 5.0 |
| C5NG | S (10.0) | — | — | — | — |
The obtained hydrogels were evaluated for their thixotropic properties. One-component hydrogels did not exhibit thixotropic behaviour; however, the hydrogels obtained from two-component mixtures C8NG/C6NG (1/1, w/w) and C8NG/C7NG (1/1, w/w) were clearly thixotropic (Fig. 1). Whereas previous reports show that the two-component hydrogel formed with C8NG/sodium dodecyl sulfate as better hydrogel,10b,12 it was only weakly thixotropic (see ESI, Fig. S3†). Therefore, these results, showed that C8NG/C6NG and C8NG/C7NG were good examples of mixing-induced thixotropy in hydrogels.
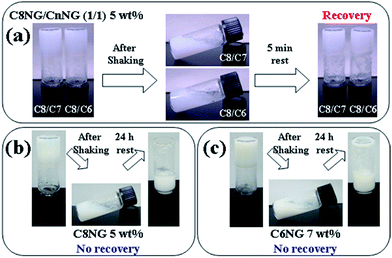 | ||
| Fig. 1 Images of hydrogels before, during and after gelation and thixotropic tests. (a) C8NG/CnNG 1/1 (w/w) 5 wt%, (b) C8NG 5 wt%, and (c) C6NG 7 wt% hydrogels. | ||
Rheological tests identified that gelation occurred with both one- and two-component mixtures, and the results obtained were similar to that observed for polymer-based gel (see ESI, Fig. S4 and S5†).17 In the frequency sweep, the existence of a gel was shown by a profile with a pseudo plateau and a storage modulus (G′) > loss modulus (G″). With increasing stress, the relationship between the G′ and G″ for each hydrogel was switched from G′ > G″ (gel state) to G′ < G″ (sol state).
To evaluate the thixotropic behaviours, the rheometry of each hydrogel was investigated by step-shear measurement (Fig. 2). The C8NG/C6NG hydrogels repeatedly showed recovery of G′ and G″ (with G′ > G″) after a large shear, whereas the hydrogel with C8NG/C7NG showed recovery only after the first deformation shear and subsequently attained a sol-like state (similar values were obtained for G′ and G″). The moduli were also recovered for hydrogels made with C8NG and C6NG (C8NG showed a two-fold recovery). However, these measurements using small amounts of gels possibly do not reflect the large-scale properties of the gel, e.g. self-support of the gel itself as observed in Fig. 1c. Fig. 2 shows the mixing induced changes in mechanical properties of CnNG(s) hydrogels qualitatively.
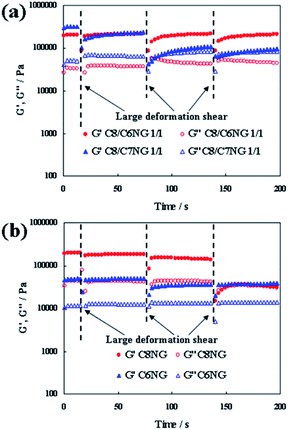 | ||
| Fig. 2 Periodic step-shear test results for hydrogels. (a) C8NG/CnNG 1/1 (w/w) 5 wt%, (b) C8NG 5 wt% and C6NG 7 wt% hydrogels. | ||
The thermal properties of one- and two-component CnNG hydrogels were characterized by differential scanning calorimetry (DSC). The analysis of DSC curves (see ESI, Table S1 and Fig S6†) indicated that sol-to-gel and gel-to-sol transition for these hydrogels involved similar ΔH's. However, the peak temperatures for the transitions in two-component hydrogels were higher than that observed for one-component gels. This suggested that hydrogelation with two-component mixtures led to the formation of a denser and finer structural network than that in one-component hydrogels.
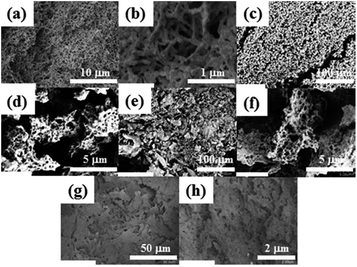
The investigation into the microstructures of hydrogels were conducted by scanning electron microscopy (SEM) analysis of the xerogels obtained from the one- and two-component hydrogels (Fig. 3). These results suggested that the two-component CnNG hydrogels had the same submicrometer order fibrous and network character as that of the one-component hydrogel. Fuhrhop et al. have provided SEM evidence for the formation of fibres of a single component in a CnNGs-based multi-gelator gels through a process of self-sorting;10c however, no evidence for self-sorting could be discerned in the data from our experiments.
X-ray diffraction (XRD) analysis of the crystal fibres of the hydrogels (see ESI, Fig. S7†) showed that similar single peaks were observed for two- and one-component hydrogels; moreover, the XRD peak positions for the hydrogels were comparable to that obtained for one-component CnNG xerogels. The position of these peaks nearly corresponded to contour molecular length and alkyl chain length of CnNG (see ESI, Fig. S8†) and suggested that these hydrogelators formed lamellar or bilayer-like structures, as shown previously.10a–c There is no direct evidence for self-sorting in these experiments as observed in Fuhrhop's mixed CnNG hydrogelators; however, such an occurrence could be masked if the peaks representing the different self-sorting structures in multi-gelator gel overlapped.
Considering self-sorting of CnNG as reported previously,10c the mixing-induced regulation of rheological properties of two-component hydrogels (i.e. prevention of crystallization, reduced CGC and onset of thixotropic behaviour) can be attributed to the possibility that the CnNGs in the two-components hydrogel reinforced each other. In this speculation, one fibre and/or network is extended by an addition of other fibre and/or network, which then enables effective crosslinking of fibres and/or network and results in inhibition of crystallization, reduction of CGC and the onset of thixotropy in two-component hydrogels.
In conclusion, we demonstrated that simply mixing two N-alkyl-D-glucamide hydrogelators induces thixotropic behaviour in the two-component hydrogel, while the one-component hydrogel did not exhibit such a behaviour. Through this study, we extended the ‘mixing’ strategy used for preparing organogels to the creation of new hydrogels with LMWGs composed of a polar head group and an alkyl substitution. We are now extending this mixing strategy further to other molecular hydrogelators.
Notes and references
- (a) A. J. Engler, S. Sen, H. L. Sweeney and D. E. Disher, Cell, 2006, 126, 677 CrossRef CAS PubMed; (b) N. A. Peppas, P. Bures, W. Leobandung and H. Ichikawa, Eur. J. Pharm. Biopharm., 2000, 50, 27 CrossRef CAS; (c) A. S. Hoffman, Adv. Drug Delivery Rev., 2002, 54, 3 CrossRef CAS; (d) R. Langer and D. A. Tirrell, Nature, 2004, 428, 487 CrossRef CAS PubMed; (e) J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337 CrossRef CAS; (f) J. J. Rice, M. M. Martino, L. De Laporte, F. Tortelli, P. S. Briquez and J. A. Hubbell, Adv. Healthcare Mater., 2013, 2, 57 CrossRef CAS PubMed.
- For book: Molecular Gels: Materials With Self-assembled Fibrillar Networks, ed. R. G. Weiss and P. Terech, Kluwer Academic Pub, 2006 Search PubMed.
- For recent reviews: (a) A. Dawn, T. Shiraki, S. Haraguchi, S.-I. Tamaru and S. Shinkai, Chem.–Asian. J., 2011, 6, 266 CrossRef CAS PubMed; (b) J. W. Steed, Chem. Commun., 2011, 47, 1379 RSC; (c) X.-Y. Yang, G.-X. Zhang and D.-Q. Zhang, J. Mater. Chem., 2012, 22, 38 RSC; (d) A. J. Jonker, D. W. P. M. Löwik and J. C. M. Van Hest, Chem. Mater., 2012, 24, 759 CrossRef CAS; (e) M. Yamanaka, J. Inclusion Phenom. Macrocyclic Chem., 2013, 77, 33 CrossRef CAS; (f) M. D. Segarra-Maset, V. J. Nebot, J. F. Miravet and B. Escuder, Chem. Soc. Rev., 2013, 42, 7086 RSC; (g) R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519 CrossRef CAS PubMed; (h) M. Cametti and Z. Džolić, Chem. Commun., 2014, 50, 8273 RSC.
- For recent examples of stimuli-responsive LMWG gels: (a) A. Dawn, T. Shiraki, H. Ichikawa, A. Takada, Y. Tahakashi, Y. Tsuchiya, L. T. N. Lien and S. Shinkai, J. Am. Chem. Soc., 2012, 134, 2161 CrossRef CAS PubMed; (b) S.-Y. Dong, B. Zheng, D.-H. Xu, X.-H. Yan, M.-M. Zhang and F.-H. Huang, Adv. Mater., 2012, 24, 3191 CrossRef CAS PubMed; (c) Z.-X. Liu, Y. Feng, Z.-C. Yan, Y.-M. He, C.-Y. Liu and Q.-H. Fan, Chem. Mater., 2012, 24, 3751 CrossRef CAS; (d) X.-Z. Yan, D.-H. Xu, X.-D. Chi, J.-Z. Chen, S.-Y. Dong, X. Ding, Y.-H. Yu and F.-H. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed; (e) S. K. Samanta and S. Bhattacharya, J. Mater. Chem., 2012, 22, 25277 RSC; (f) P.-C. Xue, J.-B. Sun, Q.-X. Xu, R. Lu, M. Takafuji and H. Ihara, Org. Biomol. Chem., 2013, 11, 1840 RSC; (g) R. Ochi, K. Kurotani, M. Ikeda, S. Kiyonaka and I. Hamachi, Chem. Commun., 2013, 49, 2115 RSC; (h) Y. Ohsedo, M. Miyamoto, A. Tanaka and H. Watanabe, New J. Chem., 2013, 37, 2874 RSC; (i) Y. Jiang, F. Zeng, R.-Y. Gong, Z.-X. Guo, C.-F. Chen and X.-B. Wan, Soft Matter, 2013, 9, 7538 RSC; (j) J. T. van Herpt, M. C. A. Stuart, W. R. Browne and B. L. Feringa, Langmuir, 2013, 29, 8763 CrossRef CAS PubMed; (k) P. Fatás, J. Bachl, S. Oehm, A. I. Jiménez, C. Cativiela and D. D. Díaz, Chem.–Eur. J., 2013, 19, 8861 CrossRef PubMed; (l) M. Ikeda, T. Tanida, T. Yoshii, K. Kurotani, S. Onogi, K. Urayama and I. Hamachi, Nat. Chem., 2014, 6, 511 CrossRef CAS PubMed; (m) R. Yang, S. Peng and T. C. Hughes, Soft Matter, 2014, 10, 2188 RSC; (n) S. Basak, J. Nanda and A. Banerjee, Chem. Commun., 2014, 50, 3004 RSC.
- (a) A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002 CrossRef CAS PubMed; (b) F. Zhao, M. L. Ma and B. Xu, Chem. Soc. Rev., 2009, 38, 883 RSC; (c) J. B. Matson and S. I. Stupp, Chem. Commun., 2012, 48, 26 RSC.
- (a) S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766 CrossRef CAS PubMed; (b) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed.
- (a) H. A. Barnes, J. Non-Newtonian Fluid Mech., 1997, 70, 1 CrossRef CAS; (b) J. Mewis and N. J. Wagner, Adv. Colloid Interface Sci., 2009, 147–148, 214 CrossRef CAS PubMed.
- For recent examples of thixotropic LMWG gels: (a) A. A. Sobczuk, Y. Tsuchiya, T. Shiraki, S.-I. Tamaru and S. Shinkai, Chem.–Eur. J., 2012, 18, 2832 CrossRef CAS PubMed; (b) N. Yan, Z.-Y. Xu, K. K. Diehn, S. R. Raghavan, Y. Fang and R. G. Weiss, Langmuir, 2013, 29, 793 CrossRef CAS PubMed; (c) Z.-Y. Xu, J.-X. Peng, N. Yan, H. Yu, S.-S. Zhang, K.-Q. Liu and Y. Fang, Soft Matter, 2013, 9, 1091 RSC; (d) D. Higashi, M. Yoshida and M. Yamanaka, Chem.–Asian. J., 2013, 8, 2548 CrossRef PubMed; (e) Z.-F. Sun, Z.-Y. Li, Y.-H. He, R.-J. Shen, L. Deng, M.-H. Yang, Y.-Z. Liang and Y. Zhang, J. Am. Chem. Soc., 2013, 135, 13379 CrossRef CAS PubMed; (f) H. Hoshizawa, Y. Minemura, K. Yoshikawa, M. Suzuki and K. Hanabusa, Langmuir, 2013, 29, 14666 CrossRef CAS PubMed.
- (a) Y. Ohsedo, M. Miyamoto, H. Watanabe, M. Oono and A. Tanaka, Bull. Chem. Soc. Jpn., 2013, 86, 671 CrossRef CAS; (b) Y. Ohsedo, H. Watanabe, M. Oono and A. Tanaka, Chem. Lett., 2013, 42, 363 CrossRef CAS; (c) Y. Ohsedo, H. Watanabe, M. Oono and A. Tanaka, RSC Adv., 2013, 3, 5903 RSC; (d) Y. Ohsedo, M. Oono, A. Tanaka and H. Watanabe, New J. Chem., 2013, 37, 2250 RSC; (e) Y. Ohsedo, M. Taniguchi, M. Oono, K. Saruhashi and H. Watanabe, RSC Adv., 2014, 4, 35484 RSC.
- (a) J.-H. Fuhrhop, P. Schnieder, J. Rosenberg and E. Boekema, J. Am. Chem. Soc., 1987, 109, 3387 CrossRef CAS; (b) J.-H. Fuhrhop, P. Schnieder, E. Boekema and W. Helfrich, J. Am. Chem. Soc., 1988, 110, 2861 CrossRef CAS; (c) J.-H. Fuhrhop and C. Boettcher, J. Am. Chem. Soc., 1990, 112, 1768 CrossRef CAS; (d) J.-H. Fuhrhop, S. Svenson, C. Boettcher, E. Rössler and H.-M. Vieth, J. Am. Chem. Soc., 1990, 112, 4307 CrossRef CAS.
- T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401 CrossRef CAS PubMed.
- L. E. Buerkle, R. Galleguillos and S. J. Rowan, Soft Matter, 2011, 7, 6984 RSC.
- S.-T. Sun and P.-Y. Wu, Soft Matter, 2011, 7, 6451 RSC.
- C. J. Capicciotti, M. Leclère, F. A. Perras, D. L. Bryce, H. Paulin, J. Harden, Y. Liu and R. N. Ben, Chem. Sci., 2012, 3, 1408 RSC.
- For reviews: (a) A. R. Hirst and D. K. Smith, Chem.–Eur. J., 2005, 11, 5496 CrossRef CAS PubMed; (b) C. A. Dreiss, Soft Matter, 2007, 3, 956 RSC; (c) L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089 RSC.
- For recent examples of multi-component LMWG gels: (a) L. Meazza, J. A. Foster, K. Fucke, P. Metrangolo, G. Resnati and J. W. Steed, Nat. Chem., 2013, 5, 42 CrossRef CAS PubMed; (b) P.-C. Xue, R. Lu, R. Zhang, J.-H. Jia, Q.-X. Xu, T.-R. Zhang, M. Takafuji and H. Ihara, Langmuir, 2013, 29, 417 CrossRef CAS PubMed; (c) M. M. Smith, W. Edwards and D. K. Smith, Chem. Sci., 2013, 4, 671 RSC; (d) S. K. Samanta and S. Bhattacharya, Chem. Commun., 2013, 49, 1425 RSC; (e) W. Edwards and D. K. Smith, J. Am. Chem. Soc., 2013, 135, 5911 CrossRef CAS PubMed; (f) A. J. Kleinsmann and B. J. Nachtsheim, Chem. Commun., 2013, 49, 7818 RSC; (g) K.-Q. Liu and J. W. Steed, Soft Matter, 2013, 9, 11699 RSC; (h) H. Kar and S. Ghosh, Chem. Commun., 2014, 50, 1064 RSC; (i) W. Edwards and D. K. Smith, J. Am. Chem. Soc., 2014, 136, 1116 CrossRef CAS PubMed.
- G. M. Kavanagh and S. B. Ross-Murphy, Prog. Polym. Sci., 1998, 23, 533 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08345h |
| This journal is © The Royal Society of Chemistry 2014 |