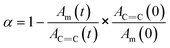Relationship between crosslinking and ordering kinetics for the fabrication of soft templated (FDU-16) mesoporous carbon thin films†
Yuanzhong Zhang,
Zhe Qiang and
Bryan D. Vogt*
Department of Polymer Engineering, University of Akron, Akron, OH 44325, USA. E-mail: vogt@uakron.edu
First published on 10th September 2014
Abstract
Although the fabrication of mesoporous carbon films through the cooperative assembly of phenolic resin oligomers (resol) and block copolymers is well-established, these methods generally rely upon simply following protocols developed for analogous bulk powders despite growing evidence of significant differences between thin films and bulk powders. Here, we examine the chemical evolution of resol through spectroscopically tracking the methylol content for films with and without a common templating agent (Pluronic F127). At lower temperature (100 °C and 120 °C), the crosslinking rate is not impacted by the presence of the template, while the addition of the Pluronic template decreases the reaction rate at higher temperatures (140 °C and 160 °C). At all conditions examined, the crosslinking kinetics of resol can be fit using the Jander model. in situ grazing incidence small angle X-ray scattering illustrates that the mesostructural evolution is not highly correlated with the chemical changes during crosslinking. With this knowledge, a shortened processing schedule (3 h total) was devised in place of the standard 24 h thermopolymerization without adversely impacting the ordered structure of the mesoporous film and this protocol significantly increases (almost double) the porosity of the film.
1. Introduction
The combination of high specific surface area, electrical and thermal conductivity, and chemical stability makes porous carbon materials attractive for numerous applications, including adsorption separations,1 catalyst support,2 and electrodes for energy storage/generation.3 Control of the pore size and its distribution can be highly advantageous for some applications;4 templated ordered mesoporous carbons (OMCs) provide one route to enable well defined pore size, geometry, and connectivity. Two distinct routes for their fabrication are hard-templating (nanocasting)5 and the soft-templating6 methods. Initially, OMCs were fabricated by hard-templating,7 but recently significant attention has been paid to the direct formation of the desired ordered mesostructures through soft templating with the development of systems based on the cooperative assembly of surfactants with either resorcinol–formaldehyde8 or oligomeric phenolic resins9 (resol). Both of these systems are readily extendable to the fabrication of mesoporous carbon films,10–12 which opens up additional potential applications13 or improved properties14 for these mesoporous materials. However, the synthesis conditions for these thin films typically are directly derived from the bulk powders without insight into the associated processes involved in transitioning from solution to the final carbon film.One of the more popular systems for the soft templating synthesis is the FDU-family of mesoporous carbons based on the cooperative assembly of resol and commercial Pluronic non-ionic surfactants, based on poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide).15–17 Examination of films for this system has revealed several differences in comparison to the synthesis of bulk powders: the compositional window for the ordered phases can be shifted,11 the morphology can be dependent on the film thickness,18 and most strikingly that the ordered structure evolves from disorder on heating through a thermally induced self-assembly (TISA) process.19 These differences suggest that the best processing conditions for the fabrication in films and powders will be different, but to date these syntheses have utilized the same long thermopolymerization process (120 °C for 24 h) as initially developed to optimize the synthesis of FDU-16.16 We have illustrated previously that the conditions utilized to fabricate an ordered mesoporous carbon powder do not always generate a highly ordered structure in thin films.20 Moreover with the TISA process, the self-assembly occurs as the resol is crosslinking, so there is an interplay between mobility of the resol/template system to enable ordering and the reaction rate associated with the crosslinking of the resol.
The resol-type phenolic resin is a well-investigated and commercially available carbon oligomeric precursor based on the condensation reaction of phenol and formaldehyde in a basic environment.21 For the fabrication of the FDU family of mesoporous carbons, low molecular weight resol is utilized with degree of polymerization less than 5;16 this is consistent with a 13C NMR study where methylol substitution of the phenol is the dominant reaction under basic condition in the 1st h, while condensation only becomes a major pathway at longer reaction times.22 This starting oligomer is then crosslinked through thermopolymerization to enable carbonization of the mesostructure. The thermally-induced cross-linking of resol precursor involves a series of reactions and the cross-linking mechanism depends on pH and temperature.21 Low temperature and non-basic (acidic or neutral) conditions are known to preferentially yield dimethylene ether bridges, but the crosslinking in thin films and with a non-ionic surfactant template have not been systematically investigated, although the mechanisms associated with the bulk powders have been reported.23 The dynamic properties of polymeric thin films have been shown to be altered from that of the bulk in many cases,24 including a dramatic reduction in acid-catalyzed deprotection reaction rate in polymeric photoresist,25 but other reactions have been reported to be unaltered in thin films.26
In this study, the coupling of the dynamics associated with the crosslinking and ordering processes involved in the fabrication of soft templated mesoporous thin films (FDU-16) is systematically investigated. In this study, a mixture of resol precursor and Pluronic template is cast into thin films and subsequently heated to (1) generate desired mesostructure and (2) promote the crosslinking of the resol to provide the requisite thermal stability for carbonization. However, these two processes occur simultaneously, thus control over the relative rates is necessary to enable a highly ordered structure, but low crosslinking rate would necessitate long thermopolymerization times. In order to understand these coupled processes, first the thermopolymerization of the resol is quantified using Fourier transform infrared (FTIR) spectroscopy by monitoring the concentration of methylol groups in the film. This reactive conversion to a highly crosslinked network is accompanied by densification of the film, which provides a route to confirm the reaction kinetics obtained from FTIR by tracking the thickness using in situ ellipsometry during thermopolymerization. As the resol crosslinks into a 3D network, the mesostructure is forming simultaneously; to understand the mesostructure evolution in situ grazing incidence small angle X-ray scattering (GISAXS) has been performed. These combined reaction and structure studies provide insight into the dynamic coupling between crosslinking and ordering. From these fundamental studies, an alternative thermopolymerization strategy is employed to decrease the crosslinking time from 24 h to 3 h without adversely impacting the final carbonized ordered mesostructure.
2. Experimental
2.1 Materials
Phenol (≥99%), formaldehyde (ACS reagent, 37 wt% solution in H2O, contains 10–15% methanol as stabilizer), Ethanol (≥99.5%), sodium hydroxide (≥97%), and Pluronic® F127 were all purchased from Sigma-Aldrich. Hydrochloric acid (fuming, ≥37%) was purchased from Fluka Analytical. All reagents were used as received. Double side polished (DSP, thickness: 500 μm, resistivity: 5–10 ohm cm) and single side polished (SSP, thickness: 600 μm, resistivity: 1–10 ohm cm) silicon wafers were acquired from Silicon Inc. (Boise, ID) and utilized as substrates for the films. The DSP silicon was utilized to minimize the scattering associated with backside roughness of the SSP wafers during transmission FTIR measurements.2.2 Sample preparation
A resol-type phenolic resin was synthesized following standard procedure for fabrication of the FDU family of mesoporous materials.16 This procedure involved the aqueous phase reaction of phenol and formaldehyde at molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 using NaOH as catalyst at 75 °C for 1 h to synthesize the oligomeric precursor. The reaction products were neutralized by dilute HCl (0.1 M) to pH = 7 at ambient temperature. The product was dried first by rotary evaporation at 40 °C for 6 h, followed by drying in vacuum at ambient temperature for an additional 12 h. The dried resol was then dissolved into ethanol to generate a 20 wt% solution. The NaCl precipitate was allowed to settle overnight. The supernate containing resol in ethanol solution was carefully collected without disturbing the precipitate to obtain the resol product.
2 using NaOH as catalyst at 75 °C for 1 h to synthesize the oligomeric precursor. The reaction products were neutralized by dilute HCl (0.1 M) to pH = 7 at ambient temperature. The product was dried first by rotary evaporation at 40 °C for 6 h, followed by drying in vacuum at ambient temperature for an additional 12 h. The dried resol was then dissolved into ethanol to generate a 20 wt% solution. The NaCl precipitate was allowed to settle overnight. The supernate containing resol in ethanol solution was carefully collected without disturbing the precipitate to obtain the resol product.
For understanding how cooperative assembly impacts the reaction rate of resol, a commonly utilized soft templating formulation, FDU-16, was prepared utilizing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 molar ratio of phenol (on the basis of the content in resol) to Pluronic F127.1 Additional ethanol was added to this mixture to generate a 5 wt% solids (resol + Pluronic F127) solution. For a control without the templating agent, resol was dissolved at 10 wt% in ethanol for film casting. Silicon wafers were cut into 3.5 cm × 2 cm pieces and cleaned with piranha solution (70
0.005 molar ratio of phenol (on the basis of the content in resol) to Pluronic F127.1 Additional ethanol was added to this mixture to generate a 5 wt% solids (resol + Pluronic F127) solution. For a control without the templating agent, resol was dissolved at 10 wt% in ethanol for film casting. Silicon wafers were cut into 3.5 cm × 2 cm pieces and cleaned with piranha solution (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) sulfuric acid and 30 wt% hydrogen peroxide) at 90 °C for 40 min. The wafers were rinsed with deionized water twice and dried with nitrogen gas before utilization as substrates for the FDU-16 and resol. Films of FDU-16 and resol were fabricated by spin coating at 3500 rpm for 30 s.
30 (v/v) sulfuric acid and 30 wt% hydrogen peroxide) at 90 °C for 40 min. The wafers were rinsed with deionized water twice and dried with nitrogen gas before utilization as substrates for the FDU-16 and resol. Films of FDU-16 and resol were fabricated by spin coating at 3500 rpm for 30 s.
2.3 Characterization
The film thickness (h) and refractive index (n) of FDU-16 and pure resol films on both SSP and DSP silicon wafers were measured using spectroscopic ellipsometry (VASE, J.A. Woollam Co., M-2000). In situ, isothermal measurements to determine h(t) and n(t) were performed using a heating stage (J.A. Woollam Co., HTC-100) without the cover to enable rapid placement and removal of the sample. Four temperatures (100 °C, 120 °C, 140 °C and 160 °C) were utilized in each case to elucidate temperature dependencies of the crosslinking reaction for FDU-16 and resol. In order to obtain utilizable data initially as the wafer is heated to the crosslinking temperature, the as-cast film samples (on SSP silicon wafers) were aligned on the heating stage at room temperature and subsequently removed before the stage was heated to target temperature. The in situ measurements were started when the temperature of the heating stage was stabilized and the sample was placed in contact with the heating stage. Each in situ measurement was performed for 660 min during which time the ellipsometric angles, Ψ and Δ, were collected from 246.6 cm−1 to 1689.8 cm−1. These angles were recursively fit with a Cauchy model to describe the optical properties of the polymer films using CompleteEASE (J.A. Woollam Co.). As the Cauchy model assumes no absorption and the resol develops an orange-brown color on crosslinking, the extinction coefficient k (the imaginary part of refractive index) is introduced into the model as an Urbach absorption to improve the fit quality.FTIR spectra (NICOLET is50) were obtained in transmission mode using a Deuterated TriGlycine Sulfate (DTGS) detector. For the FTIR experiments, the measurements were performed ex situ as the crosslinking rate is negligible at ambient temperature. In a typical experiment, the FTIR spectra of the FDU-16 or resol were measured prior to heating for a baseline. The sample was then heated (100 °C, 120 °C, 140 °C or 160 °C) on the same heating stage utilized for the ellipsometry measurements for a given duration (typically resulting in total heating times of 0.5, 1, 2, 4, 6, 8, 10, 20, 30, 60, 120, 180, 210, 300, 480, 660 min for a given sample). When the films were removed from the heating stage, the samples were quickly placed onto an aluminum heat sink at room temperature to quench the reaction. The FTIR spectra were then re-measured using 512 scans at a resolution of 8 cm−1. The sample was then re-heated at the same temperature for the desired duration to obtain the time dependence composition for both FDU-16 and resol. The baseline correction of the FTIR spectra was performed using OMNIC software. Peak locations were determined by utilizing the second-derivative of the spectra. Overlapping peaks were resolved using Gaussian functions in OriginPro 8.5.
In order to elucidate how the mesostructure evolves, GISAXS was employed using the X9 beamline of National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory (BNL). Both in situ measurements during heating as well as ex situ measurements at ambient temperature were performed under vacuum (≈40 Pa) using an incident X-ray beam of energy of 13.5 keV (λ = 0.0918 nm). The scattering data were collected using a charged-coupled device (CCD) detector at a distance of 4.73 m. The in situ measurements enabled tracking the temporal dependence of the ordering at two temperatures (100 °C and 120 °C), similar to the protocol employed by Bein and co-workers in their pioneering work.27 The ex situ samples were measured at multiple angles both below and above the critical angle.
3. Results and discussion
One challenge in assessing the thermopolymerization kinetics of resol is the complexity of the reactions with multiple pathways present, as illustrated in Fig. 1, which can significantly impede the analysis. During the synthesis of the resol precursor, the substitution reactions (Fig. 1a) are much faster than the subsequent condensation reactions (Fig. 1b–d) between two phenol rings. Thus, single methylol-substituted phenol rings are the predominant component in the resol precursor. The thermally-induced cross-linking of resol precursor is influenced by pH and temperature.21 Lower temperature (under acidic or neutral conditions) results in preferential formation of dimethylene ether bridge (Fig. 1b), while at higher temperature (or under basic conditions), methylene bridges form dominantly (Fig. 1d). As the standard processing condition for fabricating the FDU family16 is modest temperature (120 °C) and neutral (pH = 7), one might expect dimethylene ether bridges to be the dominant thermopolymerization pathway, which will decrease the total extent of crosslinking due to loss of formaldehyde. However irrespective of the pathway, both crosslinking reactions involve the consumption of the methylol moiety, which provides a route to quantification of the crosslinking.Fig. 2 illustrates the evolution of the FTIR spectra during crosslinking at 120 °C and 160 °C for both pure resol and its blend with Pluronic F127 for fabricating FDU-16. In examining the initial spectra for the resol and FDU-16 (time = 0 min), there are clear differences with additional peaks in the FDU-16 spectra at 1374 and 1343 cm−1 (associated with the –CH2– wagging), 1360 cm−1 (associated with the C–C stretch and –CH2– wag), and 1149 cm−1 (associated with the C–C and C–O–C stretch), 1113 cm−1 (associated with the C–O–C stretch). Most of the peaks associated with the resol are still readily distinguishable in the FDU-16, where the resol has been diluted by approximately 30% (on a mass basis). Table 1 lists the peaks and bands of interest for the quantitative assessment of the crosslinking reaction in thin films. There are several salient features in these spectra that provide evidence for the reactions illustrated in Fig. 1. The series of absorption bands between 900 cm−1 and 650 cm−1 are characteristic of the C–H vibration of aromatic ring, whose frequencies are impacted by the number of adjacent hydrogen atoms on the aromatic ring, while the nature of any substituents not significantly impacting the frequency.5,6 Therefore, changes in absorbance indicate new substitution on a previously unsubstituted positions.
 | ||
| Fig. 2 Temporal evolution of the FTIR spectra for (a) resol, (b) FDU-16 films at 120 °C and when crosslinking at 160 °C for (c) resol and (d) FDU-16 films. | ||
| Wavenumber of observed peak centers or bands (cm−1) | Functional group |
|---|---|
| 1740–1590 (Doublet) | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O substitution on aromatic ring30 O substitution on aromatic ring30 |
| 1484 | Aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching28 C stretching28 |
| 1460 | CH2 deformation vibration in –CH2–OH30 |
| 1149 | C–O–C stretching and C–C stretching in F127 (ref. 31) |
| 1113 | C–O–C stretching in F127 (ref. 31) |
| 1070–1050 | C–O–C stretching in both phenolic resin and F127 (ref. 31) |
| 1028; 1011; 995 | δ(O–H)ν(C–O) vibration of 2,4,6-trihydroxymethylphenol29 |
| 1020; 996; 990 | δ(O–H)ν(C–O) vibration of 2,4-dihydroxymethylphenol29 |
| 1003; 995 | δ(O–H)ν(C–O) vibration in primary alcohol of 2-hydroxymethylphenol29 |
| 1010 | δ(O–H)ν(C–O) vibration of 2,6-dihydroxymethylphenol29 |
| 991 | δ(O–H)ν(C–O) vibration of 4-hydroxymethylphenol |
| 963 | CH2 rock31 |
| 947 | CH2 rock and C–O–C stretching31 |
| 890 | Aromatic = C–H out-of-plane deformation vibration, 2,4,6-substituted phenol30 |
| 830 | 4- or 2,4-substituted phenol30 |
| 756 | 2-substituted phenol30 |
For the as-cast films (0 min), there are three distinct bands in the FTIR spectra at ≈890 cm−1, ≈830 cm−1, and ≈756 cm−1. The band at ≈890 cm−1 is characteristic of isolated hydrogen associated with 2,4,6-substituted phenol ring (fully substituted by the condensation with formaldehyde). The band at ≈830 cm−1 is associated with two adjacent hydrogens in the aromatic ring of there are two possible structures: the 4-monosubstituted or 2,4-disubstituted phenol rings. The band at ≈756 cm−1 is characteristic of four adjacent hydrogens in the aromatic, which is indicative of 2-monosubstituted phenol ring. As crosslinking of the resol occurs, the bands at ≈830 cm−1 and ≈756 cm−1 decrease in intensity (Fig. 2) as expected by the reaction illustrated in Fig. 1d, in which the unsubstituted ortho-reactive sites are consumed. For prior kinetic studies of the bulk resol crosslinking,28,29 this region was used for quantitative FTIR analysis, but these reactions occurred under basic conditions where reaction illustrated in Fig. 1d is dominant. However for the fabrication of mesoporous carbons,9 the resol is crosslinked under neutral pH conditions, which increases the pathways for the reaction (Fig. 1). In addition to this uncertainty in the reaction pathways, the low intensity of the peaks at 756 cm−1 and 830 cm−1 also impedes the use of these peaks for quantification with thin films. Thus, the peaks in the range between 1050 cm−1 and 950 cm−1 associated with the δ(O–H)ν(C–O) vibration of the methylol are utilized to assess the crosslinking kinetics for both resol and FDU-16 in thin films in this study. Similar features are found in the spectra for films heated at 100 °C and 140 °C as shown in the ESI.†
For quantification of the reaction extent, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in aromatic ring (1484 cm−1) is used as an invariant reference peak as this is not impacted by the cross-linking process. This peak has previously been utilized successfully for the quantitative analysis of base catalyzed crosslinking of phenolic resin.16 One caveat associated with this method is that the intensity of this peak is impacted by the substitution on benzene ring with an increase for electron donors and decrease for electron withdrawing groups.29 In this study, the films are exposed to air to enable the real time ellipsometry measurements, but this can lead to oxidation of the methylene bridges into carbonyl groups during the cross-linking process as illustrated in Fig. 1e. This carbonyl group is electron withdrawing and leads to a decrease in the intensity of the reference aromatic C
C in aromatic ring (1484 cm−1) is used as an invariant reference peak as this is not impacted by the cross-linking process. This peak has previously been utilized successfully for the quantitative analysis of base catalyzed crosslinking of phenolic resin.16 One caveat associated with this method is that the intensity of this peak is impacted by the substitution on benzene ring with an increase for electron donors and decrease for electron withdrawing groups.29 In this study, the films are exposed to air to enable the real time ellipsometry measurements, but this can lead to oxidation of the methylene bridges into carbonyl groups during the cross-linking process as illustrated in Fig. 1e. This carbonyl group is electron withdrawing and leads to a decrease in the intensity of the reference aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching peak at 1484 cm−1. In examining Fig. 2a, the intensity of this peak significantly decreases during the heating at 120 °C at the longest time examined (660 min), while the peak intensity is relatively invariant for shorter reaction times. This formation of carbonyl groups is confirmed by the doublet at 1740 cm−1 to 1590 cm−1 that only appears after 660 min. Interestingly, the oxidation of the resol occurs after 660 min at 120 °C for the FDU-16 as well (Fig. 2b), which suggests that the oxidative pathway is not impacted by the addition of Pluronic F127 to the resol. Increasing the reaction temperature to 160 °C leads to significant oxidation after 150 min as evidenced by the decrease at 1484 cm−1 and emergence of a doublet at 1740 cm−1 to 1590 cm−1 for both the resol (Fig. 2c) and FDU-16 (Fig. 2d) films.
C stretching peak at 1484 cm−1. In examining Fig. 2a, the intensity of this peak significantly decreases during the heating at 120 °C at the longest time examined (660 min), while the peak intensity is relatively invariant for shorter reaction times. This formation of carbonyl groups is confirmed by the doublet at 1740 cm−1 to 1590 cm−1 that only appears after 660 min. Interestingly, the oxidation of the resol occurs after 660 min at 120 °C for the FDU-16 as well (Fig. 2b), which suggests that the oxidative pathway is not impacted by the addition of Pluronic F127 to the resol. Increasing the reaction temperature to 160 °C leads to significant oxidation after 150 min as evidenced by the decrease at 1484 cm−1 and emergence of a doublet at 1740 cm−1 to 1590 cm−1 for both the resol (Fig. 2c) and FDU-16 (Fig. 2d) films.
The time to appreciable oxidation that can be readily assessed by FTIR decreases with increasing reaction temperature; no sign of oxidation is observed at 100 °C even after 660 min of heating, but oxidation can be assessed from the FTIR spectra starting at 660 min, 300 min and 150 min for 120 °C, 140 °C, and 160 °C, respectively. As the reference peak is altered by the oxidation, quantitative reaction analysis is only performed before any signs of oxidation is clearly present in the FTIR spectra.
In order to understand the crosslinking of resol under neutral conditions, the peaks associated with the methylol groups are quantified, but the δ(O–H)ν(C–O) vibrations for primary alcohol (methylol groups) are overlapping peaks between 950 cm−1 and 1050 cm−1. The substitution condition (ortho- and/or para-) and their hydrogen bonding lead to these complex bands, which has impeded their use in the past especially as the C–H vibration can provide kinetic information if only the direct condensation pathway is considered.3
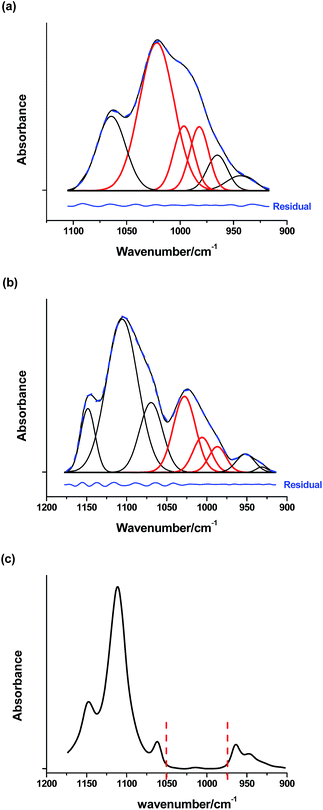
However, this assumption is likely problematic here due to the low temperature (100 °C) and neutral conditions. In addition to the δ(O–H)ν(C–O) vibrations, there are three additional peaks that are either relatively well-separated from peaks associated with the methylol (946 cm−1 and 1064 cm−1) or relatively constant in intensity (963 cm−1). In order to determine the concentration of methylol groups, these other absorption peaks are removed from the area associated with the methylol as shown in Fig. 3a for the resol film.
For FDU-16, one concern is the introduction of additional peaks due to the Pluronic F127 (Fig. 3b), which increases the complexity of the deconvolution. However, the contribution from the template is small in the region of interest as shown in Fig. 3c. With this analysis to de-convolute the absorption associated with the methylol, the extent of reaction can be directly related with the area of these peaks. As this area decreases, the hydroxyl groups have condensed, which can occur by any of the three mechanisms illustrated in Fig. 1. This sensitivity to all reaction pathways enables quantitative assessment of the crosslinking of resol under neutral conditions as a function of temperature and when mixed with the Pluronic F127 template.
In order to quantify the temporal evolution of the conversion through the methylol groups in these films, the peak area associated with the methylol content (Am) is corrected for thickness variations by normalization with the area of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching peak centered at 1484 cm−1 (AC
C stretching peak centered at 1484 cm−1 (AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The relative concentration of methylol is then normalized by this initial ratio at zero time. The conversion at time t is thus:
C). The relative concentration of methylol is then normalized by this initial ratio at zero time. The conversion at time t is thus:
Fig. 4 illustrates the temporal evolution in conversion as a function of temperature for the resol and FDU-16 films. Intriguingly, the cross-linking reactions for both resol and FDU-16 films appear to be almost identical for the two lower temperatures examined (100 °C and 120 °C) as shown in Fig. 4a and b. At higher temperatures (Fig. 4c and d), the resol crosslinking appears to outpace that of the FDU-16 as the normalized concentration of methylol decreases more rapidly for the resol only film. This latter behavior suggests that the Pluronic F127 template acts to dilute the reactive methylol groups at high temperature to decrease the overall reaction rate. However, these reactions do not appear to be impacted at lower temperatures, which suggest a more significant contribution of diffusion to the reaction kinetics at the two lower temperatures. Prior kinetic studies of the crosslinking of commercial phenolic resins in the bulk have illustrated similar characteristics.28 For crosslinking in the bulk resol, the reaction can be described as a homogeneous 1st order reaction at temperatures in excess of 140 °C, while diffusive mechanisms are required to describe the reaction kinetics at lower temperatures.28
From a qualitative perspective, these data provide insight into why the ‘optimized’ thermopolymerization temperature16 may be 120 °C as the mobility of the block copolymer template increases as the temperature increases, but crosslinking of the resol would hinder mobility requisite for ordering. At 120 °C, the conversion of methylol is approximately 50% after 16 min of heating. From prior in situ GISAXS examining FDU-15 templated by Pluronic® P123, the films begin to order in approximately 3 min at 130 °C and 5 min at 110 °C.19 This timeframe for ordering likely provides several minutes for the mesostructure to locally coarsen prior to being kinetically trapped by the cross-linking of the resol. At higher temperatures, the crosslinking likely occurs sufficiently fast to impede the ordering of the template. This behavior will be re-visited later.
One additional point is that residual methylol groups (approximately 23%) are still present in the film even after 10 hours of heating at 120 °C as shown in Fig. 4b. Increasing the temperature can decrease this residual methylol to approximately 7% after 60 min at 160 °C. This residual uncondensed fraction in the resol is a result of the hindrance of the cross-linked network that prevents the diffusion of methylol groups for further reaction. Conley32 demonstrated that full cross-linking requires heating at 400 °C in nitrogen for 1 h. However, prior studies of the crosslinking of commercial phenolic resins have suggested that a maximum conversion of 68% (minimum of 32% residual methylol) should be obtained under the conditions utilized here.28 This difference in conversion may be related to formulation additives in the commercial phenolic resin. Nonetheless, the residual methylol group can further react during calcination to contribute structure shrinkage as the template is removed.16
For a more quantitative description of the reaction kinetics, the data are fit using an integral method to the homogeneous 1st order reaction and Jander33 rate law models as these two models have been shown to describe the crosslinking of phenolic resin at high and low temperatures, respectively.28 Linear fits of each model for both resol and FDU-16 at all temperatures examined are included in the ESI.† In all cases, Jander model fits the data through a larger time window than the 1st order reaction. The best fit is shown as the lines in Fig. 4. It should be noted that the model is not able to describe the full temporal range as the reaction does not proceed to completion at the temperatures examined. As the temperature increases, the fit appears to degrade at shorter times, but this is associated with the limited conversion and more rapid approach to the plateau conversion. The relatively poor fit of the highest temperature data to the 1st order reaction model is unexpected as prior examination of the crosslinking of commercial resol in the bulk found a transition from Jander model to 1st order reaction model at approximately 140 °C.28 These differences may be related with the decreased mobility of polymers in thin films; for example, the ordering of block copolymers in thin films is significantly suppressed relative to bulk.34 Due to the differences in the best fit model, the obtained kinetic parameters at high temperature cannot be quantitatively compared for the resol. The crosslinking reaction can be monitored indirectly using the thickness change monitored by in situ ellipsometry (see ESI†). The decrease in film thickness in films is largely due to the contraction caused by cross-linking reaction. The initial rate of change in the film thickness is strongly dependent on the temperature selected as would be expected as the crosslinking rate is significantly different. The time to reach a plateau thickness is in rough agreement with the plateau in conversion (Fig. 4) for the FDU-16 films. However, the thickness of the resol films reaches a plateau values more rapidly than does the conversion, so additional processes are likely involved for the film shrinkage. Interestingly although the extent of crosslinking reaction increases with increasing crosslinking temperature, the final film thickness is not strongly dependent on the crosslinking temperature. The thickness shrinkage of the FDU-16 (resol) films after 11 h of heating, which shows a plateau in thickness, is 28% (31%), 30% (33%), 29% (31%), and 35% (34%) for 100 °C, 120 °C, 140 °C, and 160 °C, respectively. This lack of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 correlation between crosslinking extent determined chemically from FTIR and the film thickness suggests that additional crosslinking at high conversion cannot induce significant shrinkage of the framework on the macroscopic scale.
1 correlation between crosslinking extent determined chemically from FTIR and the film thickness suggests that additional crosslinking at high conversion cannot induce significant shrinkage of the framework on the macroscopic scale.
This difference between the crosslinking extent and the change in thickness of the film suggests that the evolution (ordering) of the mesostructure will only occur at low conversion. Fig. 5 illustrates snapshots of the in situ GISAXS profiles obtained during crosslinking of the FDU-16 film at 100 °C. Due to experimental constraints, the film is rapidly heated from ambient temperature to the desired crosslinking temperature, unlike the prior experiments discussed where the film is directly placed on a pre-heated stage. The first salient feature in these GISAXS profiles is the weak scattering ring initially present prior to heating. This indicates that the film is poorly ordered in the as-cast state, which is counter to the prior reports from Bein and coworkers where they found that the as cast film is completely disordered.19 However, this prior work used FDU-15 that was templated by Pluronic P123; the lower molecular weight of P123 (compared with F127 that is used here) will decrease the degree of segregation of the block copolymer, which decreases the driving force for ordering. Detailed NMR studies on the structural evolution of FDU-16 powders during crosslinking also demonstrated that these materials are initially poorly ordered as cast from solution.23 As the film is being heated to the crosslinking temperature, there is a dramatic improvement in the ordering of the film within the first 90 s. The diffuse ring is replaced by sharp diffraction peaks, which is consistent with a highly ordered mesostructure. The mesostructure continues to coarsen over the next several minutes to the higher order diffraction peaks becoming better resolved. However after approximately 4 min, the GISAXS profiles do not change appreciably based on visual inspection. For comparison, the crosslinking of FDU-16 films at the same temperature does not reach a plateau conversion until after more than 2 h of heating. This disconnect between the crosslinking kinetics and ordering kinetics suggest that at low temperatures these processes are decoupled. However, it is also known for bulk powders that there is a sweet spot for the thermopolymerization temperature to maximize the surface area,16 but extended crosslinking times have been reported in general.
In order to more quantitatively address the ordering kinetics, the evolution of the normalized intensity of the (110) reflection from GISAXS is analyzed. Fig. 6a illustrates the line scan from the 2D profile used for this analysis. There are 3 clear diffraction peaks in qx alone after thermopolymerization at 100 °C for 30 min. These peaks are indexed to the Im3m space group.16,35 FDU-16 film thermopolymerized at 120 °C for 30 min exhibit the same space group (see ESI†). As shown in Fig. 6b, the intensity of the (110) reflection grows significantly on heating with nearly an order of magnitude increase in the first 5 min at both 100 °C and 120 °C. This increase in intensity is associated with the improvement in the quality of the ordered mesostructure. However, the intensity is statistically invariant after approximately 10 min. This suggests that the mesostructure is fixed in a short time. Furthermore, the d-spacing of the mesostructure initially increases as the structure is ordering. This increase is consistent with stretching of the block copolymer chains on ordering and has been observed previously during the fabrication of both FDU-15 and FDU-16.23,27 The change in d-spacing and the scattering intensity are consistent temporally, which suggests that the mesostructure is primarily fixed after approximately 10 min of annealing. This time scale is significantly different from the crosslinking of the resol that evolves over nearly 4 h at 100 °C.
By combining the knowledge of the mesostructure from GISAXS and the crosslinking extent from FTIR, insight into the interrelationships between crosslinking and ordering for the fabrication of mesoporous carbon films can be elucidated. First, the mesostructure development occurs rapidly even at relatively low temperatures. Second, the maximum extent of conversion increases as the temperature is increased. Third, the reaction time to this plateau conversion significantly decreases as the temperature is increased. Based on prior knowledge, it is critical to utilize sufficiently low temperature to enable the system to order prior to vitrification by the crosslinking and the extent of conversion/crosslinking should be maximized to provide mechanical integrity during carbonization and to provide significant carbon yield. With the data obtained on the ordering and crosslinking, it should be possible to significantly decrease the reaction time without adversely impacting the mesostructure of the carbon film by initially heating at low temperature to generate the ordered mesostructure, followed by heating at high temperature to generate a highly crosslinked material. Fig. 7 illustrates the comparison between this accelerated heating protocol (Fig. 7a, 100 °C for 1.5 h, followed by 160 °C for 1.5 h) and the standard protocol16 (Fig. 7b, 120 °C for 24 h). In both cases, multiple orders of diffraction can be clearly seen in the GISAXS profiles and qualitative differences are not readily apparent after carbonization despite the order of magnitude difference in the crosslinking time. It is important to note that the diffraction positions are nearly identical at high qz despite the significant distortion of the lattice due to the stresses induced during carbonization.37 The mechanical integrity of the framework through the carbonization process has been illustrated to be a critical factor in maintaining the mesostructure,11,37 so the two crosslinking protocols must yield similar mechanical properties in order to produce the same mesostructure in the final carbon film.
To better illustrate the equivalence of these mesostructures, Fig. 7c shows the same line scan in qx for both materials. Two diffraction peaks are indexed to the (002) and (202) planes of the Fmmm mesostructure, but the peak positions are nearly invariant with the processing. Additionally, the apparent width of these diffraction peaks is nearly indistinguishable, which suggests that the mesostructures are very similar. To further investigate the equivalence of these mesoporous carbon films, the optical properties are measured using ellipsometry. For the mesoporous carbon obtained from the accelerated heating protocol, the refractive index (at 633 nm) is 2.01, while that from the standard heating protocol is 2.17. The significantly lower refractive index for the accelerated protocol indicates that this shortened heating enhances the porosity of the mesoporous carbon film. Assuming 2.35 as the refractive index for the amorphous carbon framework,18 the porosity can be estimated from the Bruggeman effective medium approximation to be 24.6% and 13.3% for these two films, respectively. The porosity for the standard heating regiment is consistent with previous reports for FDU-16 films.36 Thus, a highly ordered mesostructure can be obtained using an accelerated heating schedule with the added advantage of increased porosity. In the future, these multiple step heating schedules could be used to tune the porosity of the films as there is a large difference in porosity developed for this single example of a two step heating process in comparison to the standard protocols.
4. Conclusions
The crosslinking behavior of resol-type phenolic resin and FDU-16 was examined quantitatively using FTIR and by in situ ellipsometry. Crosslinking of resol in FDU-16 and resol film follow almost identical pathways at lower temperatures (100 °C and 120 °C), while FDU-16 films showed slower mechanism than resol films at higher temperatures (140 °C and 160 °C). This suggests the crosslinking was controlled by diffusion at lower temperatures while reaction controlled at higher temperatures. However, a reaction-diffusion mechanism, using the Jander model, produces an improved fit of the kinetics at all temperatures as opposed to a simple 1st order reaction. The crosslinking did not proceed to completion, but leveled out at a finite extent of reaction that increased as the temperature increased. At 160 °C, this plateau appears to be reached in less than 5 min. For FDU-16 as the crosslinking is occurring, the mesostructure is evolving, so the relative rates of ordering and reaction need to be considered. The ordering occurs in the first 10 min and is then invariant at 100 °C, which is significantly less than the time required to highly crosslink the resol at this temperature. This decoupling of the two critical processes for the fabrication of mesoporous carbon enables the design of accelerated heating protocols where low temperature is used initially to order the mesostructure, followed by a high temperature to more rapidly and thoroughly crosslink the resol. An order of magnitude decrease in the thermopolymerization time can be achieved without impacting the mesostructure on carbonization, while increasing the porosity.Acknowledgements
Partial support for this work was provided by the National Science Foundation under grant no. CBET-1144016. Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract no. DE-AC02-98CH10886. Research carried out in part at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract no. DE-AC02-98CH10886.Notes and references
- X. Zhuang, Y. Wan, C. M. Feng, Y. Shen and D. Y. Zhao, Chem. Mater., 2009, 21, 706–716 CrossRef CAS.
- A. Corma, Chem. Rev., 1997, 97, 2373–2419 CrossRef CAS PubMed.
- H. Chang, S. H. Joo and C. Pak, J. Mater. Chem., 2007, 17, 3078–3088 RSC.
- Y. Zhai, Y. Dou, D. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828–4850 CrossRef CAS PubMed.
- Z. Wang, F. Li, N. S. Ergang and A. Stein, Chem. Mater., 2006, 18, 5543–5553 CrossRef CAS.
- C. Liang, Z. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696–3717 CrossRef CAS PubMed.
- R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743–7746 CrossRef CAS.
- C. D. Liang and S. Dai, J. Am. Chem. Soc., 2006, 128, 5316–5317 CrossRef CAS PubMed.
- Y. Meng, D. Gu, F. Q. Zhang, Y. F. Shi, H. F. Yang, Z. Li, C. Z. Yu, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2005, 44, 7053–7059 CrossRef CAS PubMed.
- S. Tanaka, Y. Katayama, M. P. Tate, H. W. Hillhouse and Y. Miyake, J. Mater. Chem., 2007, 17, 3639–3645 RSC.
- L. Song, D. Feng, N. J. Fredin, K. G. Yager, R. L. Jones, Q. Wu, D. Zhao and B. D. Vogt, ACS Nano, 2010, 4, 189–198 CrossRef CAS PubMed.
- J. Schuster, R. Koehn, A. Keilbach, M. Doeblinger, H. Amenitsch and T. Bein, Chem. Mater., 2009, 21, 5754–5762 CrossRef CAS.
- L. Li, C. Song, H. Jiang, J. Qiu and T. Wang, J. Membr. Sci., 2014, 450, 469–477 CrossRef CAS PubMed.
- Y. Fang, Y. Lv, R. Che, H. Wu, X. Zhang, D. Gu, G. Zheng and D. Zhao, J. Am. Chem. Soc., 2013, 135, 1524–1530 CrossRef CAS PubMed.
- J. Wang, C. Xue, Y. Lv, F. Zhang, B. Tu and D. Zhao, Carbon, 2011, 49, 4580–4588 CrossRef CAS PubMed.
- Y. Meng, D. Gu, F. Zhang, Y. Shi, L. Cheng, D. Feng, Z. Wu, Z. Chen, Y. Wan, A. Stein and D. Zhao, Chem. Mater., 2006, 18, 4447–4464 CrossRef CAS.
- J. C. Xue, C. Henry, J. Lee and B. D. Vogt, RSC Adv., 2014, 4, 3675–3683 RSC.
- B. D. Vogt, V. L. Chavez, M. Dai, M. R. C. Arreola, L. Song, D. Feng, D. Zhao, G. M. Perera and G. E. Stein, Langmuir, 2011, 27, 5607–5615 CrossRef CAS PubMed.
- J. Schuster, R. Kohn, M. Doblinger, A. Keilbach, H. Amenitsch and T. Bein, J. Am. Chem. Soc., 2012, 134, 11136–11145 CrossRef CAS PubMed.
- A. Labiano, M. Dai, W.-S. Young, G. E. Stein, K. A. Cavicchi, T. H. Epps, III and B. D. Vogt, J. Phys. Chem. C, 2012, 116, 6038–6046 CAS.
- M. F. G. Loustalot, S. Larroque, D. Grande, P. Grenier and D. Bedel, Polymer, 1996, 37, 1363–1369 CrossRef.
- D. D. Werstler, Polymer, 1986, 27, 750–756 CrossRef CAS.
- M. Florent, C. Xue, D. Zhao and D. Goldfarb, Chem. Mater., 2012, 24, 383–392 CrossRef CAS.
- M. D. Ediger and J. A. Forrest, Macromolecules, 2014, 47, 471–478 CrossRef CAS.
- D. L. Goldfarb, M. Angelopoulos, E. K. Lin, R. L. Jones, C. L. Soles, J. L. Lenhart and W. L. Wu, J. Vac. Sci. Technol., B, 2001, 19, 2699–2704 CAS.
- J. Lee, M. Maddipatla, A. Joy and B. D. Vogt, Macromolecules, 2014, 47, 2891–2898 CrossRef CAS.
- J. Schuster, R. Koehn, M. Doeblinger, A. Keilbach, H. Amenitsch and T. Bein, J. Am. Chem. Soc., 2012, 134, 11136–11145 CrossRef CAS PubMed.
- G. Carotenuto and L. Nicolais, J. Appl. Polym. Sci., 1999, 74, 2703–2715 CrossRef CAS.
- M. F. GrenierLoustalot, S. Larroque and P. Grenier, Polymer, 1996, 37, 639–650 CrossRef CAS.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies – Tables and Charts, John Wiley & Sons, 3rd edn, 2004 Search PubMed.
- Y. L. Su, J. Wang and H. Z. Liu, Macromolecules, 2002, 35, 6426–6431 CrossRef CAS.
- R. T. Conley, J. Appl. Polym. Sci., 1965, 9, 1117–1126 CrossRef CAS.
- W. Jander, Z. Anorg. Allg. Chem., 1927, 163, 1–30 CrossRef CAS.
- C. Harrison, D. E. Angelescu, M. Trawick, Z. D. Cheng, D. A. Huse, P. M. Chaikin, D. A. Vega, J. M. Sebastian, R. A. Register and D. H. Adamson, Europhys. Lett., 2004, 67, 800–806 CrossRef CAS.
- I. W. Hamley and V. Castelletto, Prog. Polym. Sci., 2004, 29, 909–948 CAS.
- L. Song, D. Feng, C. G. Campbell, D. Gu, A. M. Forster, K. G. Yager, N. Fredin, H.-J. Lee, R. L. Jones, D. Zhao and B. D. Vogt, J. Mater. Chem., 2010, 20, 1691–1701 RSC.
- L. Y. Song, D. Feng, H. J. Lee, C. Q. Wang, Q. Y. Wu, D. Y. Zhao and B. D. Vogt, J. Phys. Chem. C, 2010, 114, 9618–9626 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: These include additional FTIR spectra associated with other temperatures, full FTIR band assignments, fits of the conversion data to Jander and 1st order reaction model, and in situ ellipsometry data. See DOI: 10.1039/c4ra08316d |
| This journal is © The Royal Society of Chemistry 2014 |