Fabrication of a visible light detector based on a coaxial polypyrrole/TiO2 nanorod heterojunction†
Wenji Zhenga,
Xiangcun Lia,
Chunxu Dongb,
Xiaoming Yana and
Gaohong He*a
aState Key Laboratory of Fine Chemicals, R&D Center of Membrane Science and Technology, Dalian University of Technology, 2 Linggong Road, Dalian, Liaoning Province, 116024 P.R. China. E-mail: hgaohong@dlut.edu.cn; Fax: +86 411 84986291; Tel: +86 411 84986291
bCollege of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000, China
First published on 4th September 2014
Abstract
A visible light detector can be fabricated using a coaxial polypyrrole/TiO2 nanorod composite. Based on the composite, the detector exhibits high responsivity up to 0.45 A W−1, impressive stability and excellent linear dependence of the photoresponse on visible light intensity. All of these properties make the polypyrrole/TiO2 nanorod composite very competitive and highly applicable in visible light detection.
Photodetectors (PDs) in the visible-light range are critical for applications in imaging sensing, light-wave communications and environmental monitoring, as well as in future memory storage and optoelectronic circuits.1,2 Recently, metal oxides, such as α-Fe2O3,3 Cu2O4 etc. have been extensively investigated as visible light detectors instead of Si-based materials, owing to their low band gap induced excellent visible light selectivity. However, these visible light detectors involve a harsh and complicated preparation process and their responsivities need to be further enhanced.
Titanium dioxide (TiO2), a wide band gap n-type semiconductor (anatase 3.2 eV and rutile 3.0 eV), has attracted wide attentions in the application of ultraviolet (UV) detection due to its distinctive UV absorption characteristics.5–8 Nevertheless, the wide band gap of TiO2 restricts the absorption of visible light to a great extent, which limits their application in the detection of visible light. Therefore it is urgent to find a photosensitizer to improve the visible light absorption of TiO2. As is well known, polypyrrole (PPy) is found to be a p-type conductive polymer with low band gap of 2.5 eV, and is widely used as a photosensitizer to improve the photocatalytic efficiency of TiO2.9,10 The photoactivity arises from the visible light absorption of PPy and the electrons transferred from the excited PPy to TiO2 and the heterojunction formed at the interface between the two components. The heterojunction can efficiently decease the recombination of electrons and holes, resulting in electrons enriched in the conductive band (CB) of TiO2.11 In the photoconductive investigation, it has been reported that heterojunction could be formed in the structures of double-layered PPy/TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11–13 and coaxial PPy/ZnO arrays.14 Compared with PPy/TiO2 double layer, the photosensitivity (the ratio of photocurrent to dark current) of coaxial PPy/ZnO arrays shows an enhancement of 2–3 orders of magnitude, due to the large heterojunction area provided by the coaxial structure. However, the visible light detectors based on the above heterojunctions have not been explored yet. Therefore, in this work, we propose a new kind of visible light detectors based on coaxial PPy/TiO2 NRs composites, in which the TiO2 NRs exhibit excellent chemical stability and high electron injection efficiency in contrast with ZnO.15 In this composite, the electron transfer process is illustrated in Fig. 1a. PPy can actively harvest the visible light matching its energy levels, and then can be excited to produce electrons in its lowest unoccupied molecular orbital (LUMO) level. Since the LOMO level of PPy is situated in an energetically higher position than the CB level of TiO2,12 those electrons could be spontaneously transferred into the CB level of TiO2 under the potential gradient. The heterojunctions formed between PPy and TiO2 play a crucial role in improving charge separation efficiency, resulting in excellent responsivity. Therefore, in this work, a series of the composites were synthesized by an in situ photo oxidative polymerization of pyrrole on the TiO2 NRs arrays surface at different reaction time, and the photodetective properties of these composites were investigated in details. The present work paves a way in the simple fabrication of a cheap and green visible light detector with high responsivity.
11–13 and coaxial PPy/ZnO arrays.14 Compared with PPy/TiO2 double layer, the photosensitivity (the ratio of photocurrent to dark current) of coaxial PPy/ZnO arrays shows an enhancement of 2–3 orders of magnitude, due to the large heterojunction area provided by the coaxial structure. However, the visible light detectors based on the above heterojunctions have not been explored yet. Therefore, in this work, we propose a new kind of visible light detectors based on coaxial PPy/TiO2 NRs composites, in which the TiO2 NRs exhibit excellent chemical stability and high electron injection efficiency in contrast with ZnO.15 In this composite, the electron transfer process is illustrated in Fig. 1a. PPy can actively harvest the visible light matching its energy levels, and then can be excited to produce electrons in its lowest unoccupied molecular orbital (LUMO) level. Since the LOMO level of PPy is situated in an energetically higher position than the CB level of TiO2,12 those electrons could be spontaneously transferred into the CB level of TiO2 under the potential gradient. The heterojunctions formed between PPy and TiO2 play a crucial role in improving charge separation efficiency, resulting in excellent responsivity. Therefore, in this work, a series of the composites were synthesized by an in situ photo oxidative polymerization of pyrrole on the TiO2 NRs arrays surface at different reaction time, and the photodetective properties of these composites were investigated in details. The present work paves a way in the simple fabrication of a cheap and green visible light detector with high responsivity.
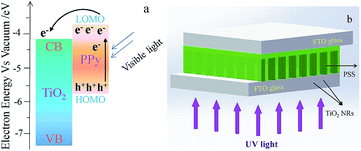 | ||
| Fig. 1 (a) The electron transfer process in PPy/TiO2 composite; (b) schematic illustration of the visible light detector. | ||
Aligned TiO2 NR arrays on FTO glass were synthesized according to our previous work.16 PPy/TiO2 NRs composites were prepared as described in ref. 17. Firstly, TiO2 NRs on FTO glass were immersed into 30 ml of 0.2 M pyrrole aqueous solution. Then UV irradiation over the reaction solution was carried out using a mercury vapor lamp (15 W) with a maximum wavelength of 365 nm. Additional monomer (0.1 M) was added every 24 h. After the reaction, the products were washed with water and ethanol and dried at 60 °C for 24 h in air. The composite is marked as PPy/TiO2-t, and t means the irradiation time. For example, PPy/TiO2-24 h represents the composite obtained after a 24 h-UV irradiation on the reaction solution.
The visible light detector was assembled by sandwiching PPy/TiO2 NRs composite between two FTO conductive glasses, as shown in Fig. 1b. The active area of the detector is 0.98 cm2. The measurements of the current–voltage (I–V) characteristics and the photoresponse of the devices were conducted with an IviumStat Electrochemical Station under the irradiation of simulated LED visible light (Ivium ISUN 12003), whose properties are shown in Fig. S1 and S2.† The visible light intensity was obtained using an optical power meter (Newport, 1916-R) and was set at 2.86 μW cm−2 for all the experiments. Different visible light intensity was achieved by adjusting the forward current.
The structures of TiO2 NRs and PPy/TiO2 NRs composites are shown in Fig. 2. From the XRD spectra, it can be seen that all the diffraction peaks could be indexed to the standard tetragonal rutile structure of TiO2 (PDF file #01-086-0147), and no impurity diffraction peaks are observed. The significantly high intensity of the (002) peak of TiO2 NRs demonstrates that the as-prepared TiO2 NR arrays are highly oriented on FTO substrates. After the modification of PPy, the relative intensities of the (002) peaks of the PPy/TiO2 NRs composites become weaker, due to screening effecting of the amorphous PPy. Moreover, the relative intensity of the (002) peaks of the PPy/TiO2 NRs composite decreases with an increase of reaction time, suggesting an enhanced amount of PPy in the composite.
The morphologies of TiO2 NRs and the composite PPy/TiO2 NRs-24 h are displayed in Fig. 3. From the cross-sectional SEM image in Fig. 3a, it is obvious that the TiO2 NRs are nearly perpendicular to the FTO substrate, and the length is about 2.4 ± 0.1 μm. Examination of individual nanorods with HRTEM shows that they are completely crystalline along their entire lengths. Lattice fringes with interplanar spacings (d110 = 3.3 ± 0.1 Å) are clearly imaged and are consistent with the rutile phase. Furthermore, the nanorods are single crystalline, as evidenced by the sharp SAED pattern of a nanorod (Fig. 3c), which is consistent with the XRD data. Fig. 3d represents the cross-sectional SEM image of PPy/TiO2 composite, it is revealed that PPy is not only grown on the top surface of TiO2 NRs, but also distributes around the TiO2 NRs, which is verified further in Fig. 3e. From Fig. 3e, a clear core–shell structure could be observed, which is then magnified in high-resolution TEM image, as shown in Fig. 3f. It is further confirmed that the core and shell are nanocrystalline TiO2 and amorphous PPy, respectively.
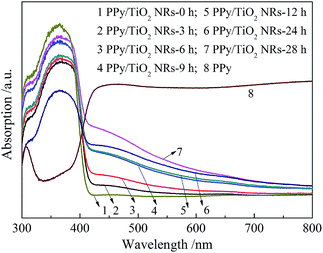
The UV-Vis absorption spectra of PPy/TiO2 composites are shown in Fig. 4. From the figure, it is clear that PPy has strong absorption in the visible light region from 400 to 800 nm, whereas pure TiO2 NRs have almost no absorption in the visible light region. After the introduction of PPy, the absorption of TiO2 NRs displays an obvious extension to the visible light region. Moreover, it is noted that the absorption intensity and range in visible light region increase gradually with increasing the reaction time, demonstrating high content of the PPy, which is consistent with the result of XRD.
The visible light response characteristics of PPy/TiO2 NRs composites are represented in Fig. 5a. As shown in this figure, there is no response for TiO2 NRs with turning visible light on and off. That is because TiO2 is visible light blind, due to its wide band gap, which is already demonstrated in the UV-Vis absorption spectrum of TiO2 NRs in Fig. 4. However, after modified by PPy, the composites exhibit obvious responsivities (defined as the photocurrent generated per unit power of incident light on the effective area of a photodetector) with visible light on. The reason is that electrons can be generated in the LUMO level of PPy and spontaneously transfer to the CB level of TiO2, resulting in the formation of photocurrent.13 When the light is off, the electrons recombine with the holes in PPy, leading to photocurrent decreasing to its original level. Moreover, it is noted that the responsivity increases from 0.04 A W−1 to 0.45 A W−1 with reaction time increasing from 3 h to 12 h. This is ascribed to the large number of electrons provided by the increasing amount of PPy. Nevertheless, when reaction time is further extended, the responsivity decreases gradually and approaches 0.15 A W−1 finally at 28 h, resulting from the block PPy layer formed on the surface of TiO2 NRs, which reduces the electron transfer rate and induces the recombination of electrons and holes.12 Therefore, it is concluded that when the reaction time reaches 12 h, the highest responsivity of the PPy/TiO2 NRs composite based visible light detectors is 0.45 A W−1, which is significantly higher than the commercial values (0.1–0.2 A W−1).8 Meanwhile, the photosensitivity of that detector is up to ∼29. The above high responsivity and photosensitivity could possibly be related to not only the electrons transferred from PPy to TiO2, but also the rectification of heterojunction between the two components. In addition, the five time response characteristics for each sample suggest that the as-designed visible light detectors are stable and repeatable, and the stability is further confirmed by the consistent current response of more than 20 cycles (shown in Fig. S3†).
In order to verify the existence of the heterojunction, Fig. 5b shows the I–V characteristics of the composite PPy/TiO2 NRs-12 h. In the dark, the rectifying ratio (defined as the ratio of photocurrent under the forward-bias to the photocurrent under the backward-bias) of the composite is about 9, indicating the formation of heterojunction at the interface. Under the visible light illumination, there is a considerable enhancement in the photocurrent under the forward-bias, leading to a rectifying ratio as high as 142. Such a good heterojunction can separate the photogenerated electrons and holes effectively, and result in an impressive responsivity.
The dependence of the photocurrent on the incident light intensity shown in Fig. 5c gives another important characteristic of the TiO2-based visible light detectors. The photocurrent increases with an increase in the visible light intensity from 0.55 to 4.44 μW cm−2. The linear fitting result shown in Fig. 5d exhibits a fabulous linear dependence of the photocurrent on the light intensity. In addition, it is worth noting that the PPy/TiO2-12 h-based visible light detector exhibits a photocurrent of 0.35 μA and a photosensitivity of 13 even at the ultralow light intensity of 0.55 μW cm−2, indicating an excellent visible light response behavior under low light intensity.
Conclusions
In summary, coaxial PPy/TiO2 NRs composites based visible light detectors with high responsivity were successfully fabricated on a FTO glass. The composites were easily synthesized by an in situ photo oxidative polymerization of pyrrole on the TiO2 NRs arrays surface, and the content of PPy was controlled by the reaction time. Morphology and structure analyses revealed that PPy were not only deposited on the top surface of TiO2 NRs, but also into the interface between them, resulting in a core–shell structure consisting of nanocrystalline TiO2 NRs and amorphous PPy. This kind of composite structures provide effective heterojunctions and strong visible light absorption in the region from 400 to 800 nm. Among the composites based visible light detectors, PPy/TiO2 NRs-12 h based one exhibits the best photodetective properties, with a responsivity of 0.45 A W−1 and a photosensitivity of 29. Furthermore, the excellent linear dependence of the photoresponse of PPy/TiO2 composite on the light intensity makes it a good candidate for visible light detection applications.Acknowledgements
This work was financially supported by the National Science Fund for Distinguished Young Scholars of China (2112562) and Fundamental Research Funds for the Central Universities (lzujbky-2013-61).Notes and references
- X. Gong, M. Tong, Y. Xia, W. Cai, J. Moon, Y. Cao, G. Yu, C. L. Shieh, B. Nilsson and A. J. Heeger, Science, 2009, 325, 1665 CrossRef CAS PubMed.
- T. Zhai, X. Fang, M. Liao, X. Xu, L. Li, B. Liu, Y. Koide, Y. Ma, J. Yao, Y. Bando and D. Golberg, ACS Nano, 2010, 4, 1596 CrossRef CAS PubMed.
- L. C. Hsu, Y. P. Kuo and Y. Y. Lia, Appl. Phys. Lett., 2009, 94, 133108 CrossRef PubMed.
- Y. Luo, L. Wang, Y. Zou, X. Sheng, L. Chang and D. Yang, Electrochem. Solid-State Lett., 2012, 15, H34 CrossRef CAS PubMed.
- X. Kong, C. Liu, W. Dong, X. Zhang, C. Tao, L. Shen, J. Zhou, Y. Fei and S. Ruana, Appl. Phys. Lett., 2009, 94, 123502 CrossRef PubMed.
- T. Y. Tsai, S. J. Chang, W. Y. Weng, C. L. Hsu, S. H. Wang, C. J. Chiu, T. J. Hsueh and S. P. Chang, J. Electrochem. Soc., 2012, 159, J132 CrossRef CAS PubMed.
- W. Wang, C. Shan, H. Zhu, F. Ma, D. Shen, X. Fan and K. Choy, J. Phys. D: Appl. Phys., 2010, 43, 045102 CrossRef.
- J. Zou, Q. Zhang, K. Huang and N. Marzari, J. Phys. Chem. C, 2010, 114, 10725 CAS.
- N. M. Dimitrijevic, S. Tepavcevic, Y. Liu, T. Rajh, S. C. Silver and D. M. Tiede, J. Phys. Chem. C, 2013, 117, 15540 CAS.
- Q. Luo, X. Li, D. Wang, Y. Wang and J. An, J. Mater. Sci., 2011, 46, 1646 CrossRef CAS.
- R. N. Bulakhe, S. V. Patil, P. R. Deshmukh, N. M. Shinde and C. D. Lokhande, Sens. Actuators, B, 2013, 181, 417 CrossRef CAS PubMed.
- Y. Jia, P. Xiao, H. He, J. Yao, F. Liu, Z. Wang and Y. Li, Appl. Surf. Sci., 2012, 258, 6627 CrossRef CAS PubMed.
- J. Wang and X. Ni, Solid State Commun., 2008, 146, 239 CrossRef CAS PubMed.
- Y. Oaki, T. Oki and H. Imai, J. Mater. Chem., 2012, 22, 21195 RSC.
- M. Quintana, T. Edvinsson, A. Hagfeldt and G. Boschloo, J. Phys. Chem. C, 2007, 111, 1035 CAS.
- W. Zheng, X. Li, G. He, X. Yan, R. Zhao and C. Dong, RSC Adv., 2014, 4, 21340 RSC.
- X. Li, G. Jiang, G. He, W. Zheng, Y. Tan and W. Xiao, Chem. Eng. J., 2014, 236, 480 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07886a |
| This journal is © The Royal Society of Chemistry 2014 |