Water desalination through armchair carbon nanotubes: a molecular dynamics study
J. Azamat*,
J. J. Sardroodi and
A. Rastkar
Molecular Simulations Lab., Azarbaijan Shahid Madani University, Tabriz, Iran. E-mail: jafar.azamat@azaruniv.edu; jafar.azamat@yahoo.com
First published on 13th November 2014
Abstract
In this paper, molecular dynamics simulations were performed to study the desalination performance of armchair carbon nanotubes (CNTs). The studied systems included (7, 7) and (8, 8) CNTs embedded in a silicon nitride membrane immersed in an aqueous ionic solution. For desalinating water, an external electrical field was applied to the systems along the axis of the nanotubes. The results indicated that the (7, 7) and (8, 8) CNTs were exclusively selective to ions. The (7, 7) CNTs selectively separated sodium ions from aqueous solution. In contrast, the (8, 8) CNTs selectively separated chlorine ions. The ion selectivity of the CNTs is justified by calculating the potential of mean force for each ion in the related system. Also, the results were confirmed using the following simulated properties: ion current, retention time of the ions, transport rate for water molecules, average density of water inside the CNTs, and radial distribution functions of ion–water. Based on the findings of this study, the studied systems can be recommended as models for the desalination of water.
1. Introduction
Water is the most important component of the world and it has a critical role in the ecosystems of the Earth. Water use has been increasing at a rate faster than that of population growth. Clean water is not available in some parts of the world and many people in the world do not have access to clean water due to natural disasters, war and weak infrastructure for purifying water. Contamination has also diminished the quality of water resources.1–3Water resources supply the required water for different types of use. Various resources are used for the production of water around the world. Saltwater is considered as one important type of water resource. When the concentration of dissolved salts increases significantly, the water will become saltwater or saline water. Saltwater resources are very useful for humans and they also save the environment from harm. Desalinated water is separate from freshwater and saline water. Oceans and seawater are the main sources of saltwater. Water desalination technology has changed and now nanotechnology is being used. However, the lack of access to clean water has called for the development and use of new technologies. The most important challenge in treating contaminated water is the cost of the required methods. It can be maintained that selecting the proper nanostructures for constructing membranes will play an important role in the effectiveness of water-desalination processes. The selective transport of ions by means of nanostructure membranes is of high significance and is regarded as a hot issue in many related research areas. The use of nanomaterials in general, and carbon nanotubes (CNTs) in particular, is considered as one of the controversial arguments in this area. CNTs, with their nano-scale diameters, can have potential applications in the desalination process.
CNTs have cylindrical nanostructures and are conceptualized by wrapping graphene.4 CNTs are characterized by a pair of indexes, (n, m). The n and m indexes are the number of unit vectors of the graphene sheet. Based on the values of these two indexes, there are three types of CNTs: zigzag (m = 0), armchair (n = m) and chiral (n ≠ m). The inner diameter of a CNT can be computed from eqn (1):5
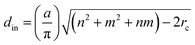 | (1) |
Since their discovery in 1991,6 CNTs have been studied extensively.7–9 Due to their special properties, CNTs have attracted researchers’ attention. The electronic structure of a CNT can be both semiconductor and metallic.10,11 Since CNTs provide a low energy solution for water treatment, they are considered as useful and efficient nanomaterials for desalinating water in membrane industries. The diameter of nanotubes is regarded as an effective parameter in transporting ions. It has been shown that the small diameter of CNTs rejects ions and only water molecules can be passed through the nanotubes.5,12,13 Some studies have experimentally indicated that water molecules can enter CNTs.14 It can be mentioned that further studies on CNTs can reveal their potential applications. Lately, there has been growing interest in studying the mechanisms of ion transport. A variety of nanotubes have been suggested as membranes for ion separation.15–19 To date, researchers have designed and fabricated ion-selective nanotubes constructed from carbon atoms.20–28
In this study, molecular dynamics (MD) simulations of (7, 7) and (8, 8) CNTs were used to study the selective removal of sodium and chloride ions by applying an external electrical field. In another work,29 ab initio MD was utilized to investigate water ionization, rather than an electrical field. The MD simulation technique can investigate the microscopic details of the desalination phenomenon. The design, production and scale-up of various processes can be simulated by this method.
2. Computational method and details
As mentioned above, (7, 7) and (8, 8) CNTs were selected in the present study. The optimized geometries of the CNTs were done at the B3LYP level of theory using 6-311G (2d, 2p) basis sets using the GAMESS-US package.30MD calculations were performed using the NAMD molecular dynamics package developed at the University of Illinois at Urbana-Champaign31 with a 1 fs time step, and they were visualized by VMD, a visualization package available from UIUC,32 as in previous works.33–36 The effective potential energy (Ueff) of the intermolecular interactions was given by the sum of Lennard-Jones 12-6 (Uvdw) and Coulomb (UC) potentials for short-range and long-range interactions, respectively, through eqn (2).
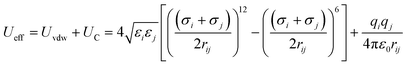 | (2) |
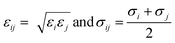 | (3) |
All analysis scripts were composed locally using both VMD and Tcl commands. The Particle Mesh Ewald algorithm39 was used for the electrostatic calculations. The MD domain consisted of a CNT fixed in a silicon nitride membrane, water molecules, and 0.5 M NaCl aqueous solution (see Fig. 1)
In experiments, nanotubes are often embedded in silicon nitride40 or a polystyrene film.41,42 In MD simulations, nanotubes have been embedded in a variety of matrices, including silicon nitride,43 lipid bilayers,44 and graphene bilayers.45,46 The length of the CNTs was 15 Å and their radii were 4.755 Å and 5.445 Å for the (7, 7) and (8, 8) CNTs, respectively. The simulation box for all runs was 3.5 × 4 × 5 nm3. The system was first minimized for 1 ns, and then equilibrated with MD for 4 ns. An electrical field was used for all the systems, which was defined through eqn (4).
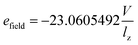 | (4) |
| Atom | Partial charges | σ (Å) | ε (kcal mol−1) |
|---|---|---|---|
| Carbon | 0 | 3.369 | 0.0607 |
| Nitrogen | −0.5607 | 3.559 | 0.190 |
| Silicon | 0.7710 | 3.804 | 0.310 |
| Sodium | +1.00 | 2.439 | 0.0874 |
| Chlorine | −1.00 | 4.477 | 0.0356 |
The silicon nitride membrane and CNTs were restrained with a harmonic constraint, while the water molecules and ions were allowed to move freely. The ion current for the studied systems was computed using eqn (5).
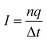 | (5) |
The contrasting ion selectivity of the CNTs can be explained by the potential of mean force (PMF).51 The PMF of the specific ion moving through the nanotube was determined using umbrella sampling, in which a harmonic biasing potential was used, i.e.,
| (Kz/2)(z − zi)2 | (6) |
This was applied to a test ion, where z refers to the axial coordinates of the ion defined from the centre of one of the pores, zi denotes the target ion positions, and Kz is the corresponding force constants. The ions were moved through positions from −7.5 Å to 7.5 Å. in 0.5 Å increments and the z component was held using a harmonic constraint of 12.5 kcal mol−1 Å−2, whereas the ion was free to move radially. This harmonic constraint was chosen to give sufficient overlap between each window and its neighbours while constraining the ions to ensure sufficient sampling of the entire reaction coordinate. Prior to all simulations, the test ion(s) was held fixed for 10 ps to allow water to equilibrate around it. Each sampling window was run for 1 ns. The ion coordinates were obtained during each umbrella sampling run of 1 ns, and the data were analysed using the weighted histogram analysis method52 (WHAM), to obtain the PMF.
3. Results and discussion
To investigate the desalination phenomenon, the MD simulations method was selected, which is considered to be an appropriate tool for the purpose of the study. The investigated system included 0.5 M NaCl aqueous solution and (7, 7) and (8, 8) CNTs. Also, an external electrical field was applied to examine the desalination process. Under the influence of this electrical field, a sodium or chlorine ion permeates from the appropriate CNT. In this study, the following parameters were investigated:• Ionic current.
• Normalized transport rate of water with respect to the number of transported ions.
• Hydrogen bonds inside the CNTs.
• Average density of water inside the CNTs.
• Ion retention time.
• Ion–water radial distribution functions.
Although the examined CNTs have a radius which is large enough for Na+ and Cl− ions to enter, the results of MD simulations indicated that the ions only permeated through these nanotubes after applying the electrical field. The transportation directions of the two kinds of ions were different because the electric field was just in one direction, therefore the sodium and the chlorine were transported in opposite directions. The results of this study reveal that one Na+ ion entered the (7, 7) CNT and was able to go through the entire length of the nanotube and get out of it. In contrast, none of the Cl− ions were even able to enter the (7, 7) CNT. In the case of the (8, 8) CNT, the opposite phenomenon occurs.
The silicon nitride membrane atoms in the immediate neighbourhood of the CNT’s control the type of ions entering into the CNTs. This arises from the orientation of the dipole moment of water molecules due to the electrostatic interactions between the membrane atoms and the oxygen or hydrogen atoms of water. The direction of the dipole moment vector of the water molecules orients to the walls of the (7, 7) CNTs, while it orients toward the axis of the (8, 8) CNTs. This different orientation is caused by the membrane nitrogen and silicon atoms surrounding the (7, 7) and (8, 8) CNTs, respectively. In the absence of CNTs, the considered ions are adsorbed in the pore inside the membrane. Water desalination is a phenomenon dependent on both CNT diameter and the type of surrounding atoms belonging to the membrane.
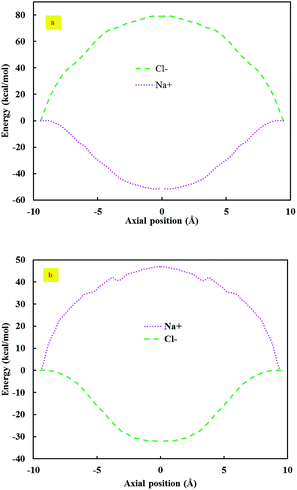
The phenomenon of ion selectivity in the respective systems was examined by calculating the potential of mean force (PMF); i.e., the free energy profile for a given ion as it was moved along the z axis of the system. Fig. 2 represents the PMF for the considered ions. As it can be observed from this figure, there was an energy barrier in the (7, 7) CNT for Cl− ions, which inhibited the permeation of Cl−. In the case of the (8, 8) CNT, there was an energy barrier for Na+ ions.
In the conducted simulations, the PMF increased at the pore openings and reached its maximum value at the pore centre inside the nanotube. This was due to the interactions between the anion, the CNT and the silicon nitride membrane. This was in complete agreement with the results of the simulations. The simulation results acknowledged that since the PMF had a high free energy barrier for the anions, they could not penetrate the (7, 7) CNT.
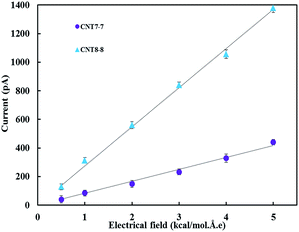
Fig. 3 shows the current–electrical field profile. It was observed that the current increased linearly with the application of electrical field. By fitting a linear regression to the current–electrical field curve, the sodium conductance was calculated to be 128.2 pS in the (7, 7) CNT and the chloride conductance was calculated to be 421.3 pS in the (8, 8) CNT.
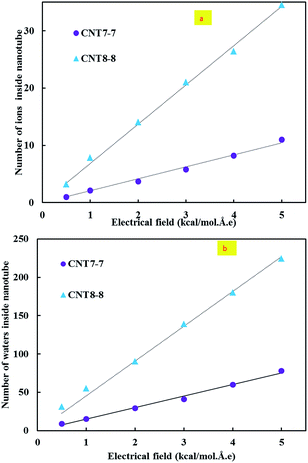
Also, the number of selective ions and water molecules passing through the CNTs increased linearly with the application of the electrical field, which is depicted in Fig. 4.
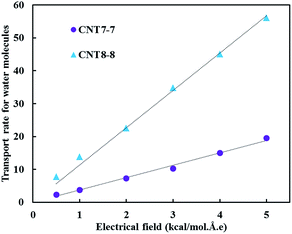
The transport rate of the water molecules (TRW) through the CNTs is defined as the ratio of the average number of passing water molecules to the simulation time. Fig. 5 shows that the TRW for the (8, 8) CNT is greater than that for the (7, 7) CNT, which is due to the larger radius of the (8, 8) CNT.
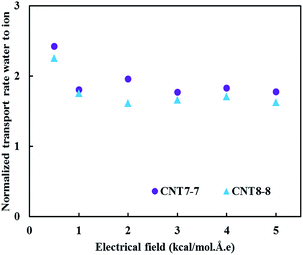
Fig. 6 shows the normalized TRW with respect to the number of transported ions. These results substantiate that the normalized TRW (transported water for one permeated ion) was almost independent of the applied electrical field.
Also, as it can be seen in this figure, this parameter in the (7, 7) CNT is larger than that of the (8, 8) CNT. This trend indicated that with respect to each ion passing through the CNT, more water molecules passed through the (7, 7) CNT in comparison to the (8, 8) CNT. This phenomenon also acknowledged that in the radial distribution function (RDF), the peak intensity of sodium ions is higher than that of chloride.
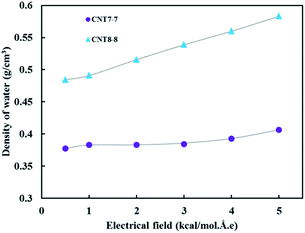
Fig. 7(a) demonstrates the number of hydrogen bonds inside the CNTs under the application of an electrical field. In this system, the number of hydrogen bonds increases as the applied electrical field increases. Since the applied electrical field increases, more water passes through the CNTs. It should be noted that the magnitude of increase in the (8, 8) CNT is higher than that of the (7, 7) CNT, which is attributed to the larger radius of the (8, 8) CNT. Another parameter that confirmed this trend is the time average of the normalized hydrogen bonds with respect to the number of inner water molecules in the applied electrical field (see Fig. 7(b)). As it was hypothesized, this parameter also increases when increasing the applied electrical field. The average density of water inside the CNTs over 4 ns simulation (see Fig. 8) also confirmed this trend.
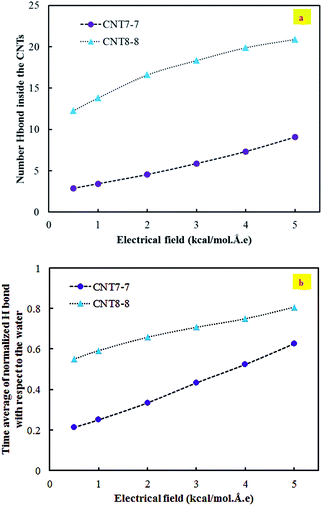 | ||
| Fig. 7 (a) The number of hydrogen bonds inside the CNTs in the applied electrical field. (b) The time average of the normalized hydrogen bonds with respect to the number of inner water molecules. | ||
Table 2 displays the retention time of the ions, which is equal to the time it takes to pass one ion through the nanotube as a function of the applied electrical field.
| Electrical field (kcal mol−1 Å−1 e−1) | Retention time of ions (ns) | |
|---|---|---|
| Na+ in (7, 7) CNT | Cl− in (8, 8) CNT | |
| 0.5 | 1.996 | 1.532 |
| 1 | 1.136 | 0.451 |
| 2 | 0.583 | 0.267 |
| 3 | 0.258 | 0.097 |
| 4 | 0.071 | 0.050 |
| 5 | 0.0108 | 0.0111 |
The retention time will be less if ions pass quickly through the CNTs, and this will speed up water desalination. This table shows that the larger the electrical field, the smaller the retention time. As it might be expected, the retention time is larger for sodium ions in the (7, 7) CNT than for chlorine ions in the (8, 8) CNT. Given the PMF curves, it can be observed that sodium ions, when compared with chloride ions, are more inclined to stay in the potential well. This is why the rate of permeation of sodium ions was less than that of chlorine ions. Moreover, these ions spend more time inside the (7, 7) CNT.
To describe the structure of each ion in the simulation box, the RDF was calculated from the trajectory files. Fig. 9 represents the RDF between ions and water molecules under the applied electrical fields. Due to the repulsive forces between atom types, at a short distance, RDF is zero. The locations of the maximum and minimum of the peaks were similar in all electrical fields. However, the intensity of the peaks was different in each electrical field, which indicates the change in the hydration number of the ions (sodium or chlorine) in each electrical field. The intense first peaks of the RDFs can be considered as evidence of the formation of a well-pronounced first coordination (solvation) shell around the ions.
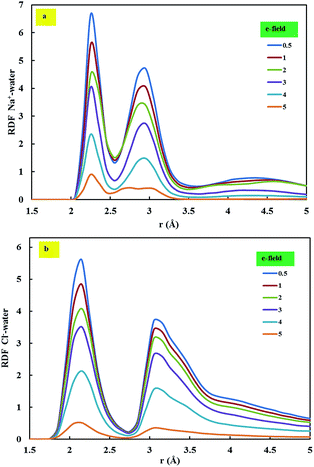 | ||
| Fig. 9 RDF for ion–water inside the CNTs at the applied electrical field: (a) (7, 7) CNT; (b) (8, 8) CNT. | ||
Fig. 9(a) displays the sodium–water RDF inside the (7, 7) CNTs, while Fig. 9(b) illustrates the RDF of chlorine–water inside the (8, 8) CNTs. As shown in these figures, the peak intensity changes when changing the applied electric field. This behaviour can be explained by the retention time of the ions. Under lower electrical fields, the ion spends extra time in its hydration shell; that is, it has a longer retention time, therefore the ion–water RDF would be intensified. A close examination of Table 2 and Fig. 9 reveals that the order of the RDF for the ions is the same as that of the retention times. In other words, the RDF with a higher peak corresponds to the longer retention time. Furthermore, the RDFs show broad second peaks with similar intensities. This indicates that the second hydration shells of the ions can be identified, but they are not defined as the first hydration shell.
Fig. 10 illustrates the z position of Na+ during the simulation time under low and high electric fields. As shown in this figure, there is an overlap between the retention times of the ions in the lower electrical field, but there is no overlap in the higher electrical field.
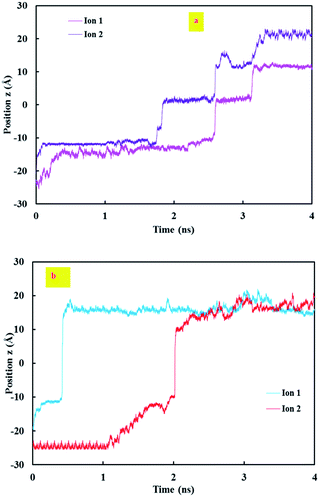 | ||
| Fig. 10 The z position of Na+ during the simulation time (a) in the lower electrical field and (b) in the higher electrical field. | ||
In other words, under the application of lower electric fields, one ion gets into the CNT and does not get out of it until a second ion enters. However, under a higher electric field, ions cross the CNT without the assistance of other ions.
In the present study, we made an attempt to explain the time evolutions in the water molecules under an applied electrical field. In doing so, the time variations of the force applied on the particles (as the electrical field) in the simulation were utilized. At the outset of the simulation, as a consequence of the deviation of the system from the equilibrium, these force components (such as other thermodynamic and mechanical quantities) have relatively large fluctuations with values which move towards optimum values. These values are directed by the integration or optimization algorithm. The applied forces on the particles are accompanied by other factors (band, angle, electrostatic and coulomb forces), which have been accounted for by solving the equations of movement and integration with respect to time. As the simulation runs and the system goes to the equilibrium state, the magnitude of the applied electrical field approaches the optimal, equilibrium value during the simulation and their thermodynamic values are regarded as the average value at the simulation time period.
4. Conclusions
For desalinating water, a series of MD simulations were conducted to investigate the ion selective permeation events through (7, 7) and (8, 8) CNTs. The results of the MD simulations indicated that ion selectivity was realized by the CNTs under the effect of using an electrical field. The results of the study revealed that one Na+ ion entered the (7, 7) CNT and managed to go through the entire length of the nanotube and then get out of it. In contrast, the Cl− ions were not able to even enter the (7, 7) CNT. In the case of the (8, 8) CNT, the opposite phenomenon occurred. Indeed, it can be argued that the simplicity of CNT structures is a significant advantage for manufacturing an ion-selective nanodevice. It can be concluded that the application of electrical field can have an impact on the following properties of the studied systems: PMF, ionic current, ion retention time, water density, TRW and RDF. Finally, it can be concluded that the results of this study highlight the capability of CNTs with respect to water desalination technology.Acknowledgements
The authors thank the Azarbaijan Shahid Madani University and Iranian Nanotechnology Initiative Council for their support.Notes and references
- M. L. Davis, Water and wastewater engineering: design principles and practice, McGraw-Hill, New York, 2011 Search PubMed.
- N. K. Shammas and L. K. Wang, Water and wastewater engineering: water supply and wastewater removal, Wiley, Hoboken, 2011 Search PubMed.
- R. L. Droste, Theory and practice of water and wastewater treatment, J. Wiley, New York, 1997 Search PubMed.
- A. Thess, R. Lee, P. Nikolaev, H. Dai, P. Petit, J. Robert, C. Xu, Y. H. Lee, S. G. Kim, A. G. Rinzler, D. T. Colbert, G. E. Scuseria, D. Tománek, J. E. Fischer and R. E. Smalley, Science, 1996, 273, 483–487 CAS.
- B. Corry, J. Phys. Chem. B, 2008, 112, 1427–1434 CrossRef CAS PubMed.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- J.-Y. Guo, C.-X. Xu, F.-Y. Sheng, Z.-L. Jin, Z.-L. Shi, J. Dai and Z.-H. Li, Quantum Matter, 2013, 2, 181–186 CrossRef CAS PubMed.
- M. Meyyappan, Carbon nanotubes: science and applications, CRC Press, 2004 Search PubMed.
- S. Kar, R. C. Bindal and P. K. Tewari, Nano Today, 2012, 7, 385–389 CrossRef CAS PubMed.
- M. S. Dresselhaus, G. Dresselhaus and P. Avouris, Carbon nanotubes: synthesis, structure, properties, and applications, Springer, New York, 2001 Search PubMed.
- M. Endo, S. Iijima and M. S. Dresselhaus, Carbon nanotubes, Pergamon, Oxford, 1st edn, 1996 Search PubMed.
- J. Azamat and J. Sardroodi, Monatsh. Chem., 2014, 145, 881–890 CrossRef CAS.
- J. J. Sardroodi, J. Azamat, A. Rastkar and N. R. Yousefnia, Chem. Phys., 2012, 403, 105–112 CrossRef CAS PubMed.
- B. Corry, Energy Environ. Sci., 2011, 4, 751–759 CAS.
- D. A. Doshi, N. K. Huesing, M. Lu, H. Fan, Y. Lu, K. Simmons-Potter, B. G. Potter, A. J. Hurd and C. J. Brinker, Science, 2000, 290, 107–111 CrossRef CAS.
- D. Stein, M. Kruithof and C. Dekker, Phys. Rev. Lett., 2004, 93, 035901 CrossRef.
- K. Leung, S. B. Rempe and C. D. Lorenz, Phys. Rev. Lett., 2006, 96, 095504 CrossRef.
- P. S. Goh, A. F. Ismail and B. C. Ng, Desalination, 2013, 308, 2–14 CrossRef CAS PubMed.
- C. H. Ahn, Y. Baek, C. Lee, S. O. Kim, S. Kim, S. Lee, S.-H. Kim, S. S. Bae, J. Park and J. Yoon, J. Ind. Eng. Chem., 2012, 18, 1551–1559 CrossRef CAS PubMed.
- A. Kalra, S. Garde and G. Hummer, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10175–10180 CrossRef CAS PubMed.
- A. Waghe, J. C. Rasaiah and G. Hummer, J. Chem. Phys., 2002, 117, 10789–10795 CrossRef CAS PubMed.
- C. F. Lopez, S. O. Nielsen, P. B. Moore and M. L. Klein, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 4431–4434 CrossRef CAS PubMed.
- R. Das, M. E. Ali, S. B. A. Hamid, S. Ramakrishna and Z. Z. Chowdhury, Desalination, 2014, 336, 97–109 CrossRef CAS PubMed.
- C. Y. Lee, W. Choi, J.-H. Han and M.S. Strano, Science, 2010, 329, 1320–1324 CrossRef CAS PubMed.
- T. A. Hilder, D. Gordon and S.-H. Chung, Biophys. J., 2010, 99, 1734–1742 CrossRef CAS PubMed.
- M. E. Suk, A. V. Raghunathan and N. R. Aluru, Appl. Phys. Lett., 2008, 92 Search PubMed.
- F. Fornasiero, H. G. Park, J. K. Holt, M. Stadermann, C. P. Grigoropoulos, A. Noy and O. Bakajin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17250–17255 CrossRef CAS PubMed.
- M. Majumder, N. Chopra, R. Andrews and B. J. Hinds, Nature, 2005, 438, 44 CrossRef CAS.
- M. K. Tsai, J. L. Kuo and J. M. Lu, Phys. Chem. Chem. Phys., 2012, 14, 13402–13408 RSC.
- M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis and J. A. Montgomery, J. Comput. Chem., 1993, 14, 1347–1363 CrossRef CAS.
- L. Kalé, R. Skeel, M. Bhandarkar, R. Brunner, A. Gursoy, N. Krawetz, J. Phillips, A. Shinozaki, K. Varadarajan and K. Schulten, J. Comput. Phys., 1999, 151, 283–312 CrossRef.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS.
- J. Azamat and J. J. Sardroodi, J. Comput. Theor. Nanosci., 2014, 11, 2611–2617 CrossRef PubMed.
- J. Azamat, J. J. Sardroodi and A. Rastkar, Desalin. Water Treat., 2014, 1–9 CrossRef CAS.
- J. Azamat, A. Khataee and S. Joo, J. Mol. Model., 2014, 20, 1–9 CrossRef CAS PubMed.
- J. Azamat, A. Khataee and S. W. Joo, J. Mol. Graphics Modell., 2014, 53, 112–117 CrossRef CAS PubMed.
- R. J. Mashl, S. Joseph, N. R. Aluru and E. Jakobsson, Nano Lett., 2003, 3, 589–592 CrossRef CAS.
- Y. Shim, Y. Jung and H. J. Kim, Phys. Chem. Chem. Phys., 2011, 13, 3969–3978 RSC.
- T. Darden, D. York and L. Pedersen, J. Chem. Phys., 1993, 98, 10089–10092 CrossRef CAS PubMed.
- J. K. Holt, H. G. Park, Y. Wang, M. Stadermann, A. B. Artyukhin, C. P. Grigoropoulos, A. Noy and O. Bakajin, Science, 2006, 312, 1034–1037 CrossRef CAS PubMed.
- B. J. Hinds, N. Chopra, T. Rantell, R. Andrews, V. Gavalas and L. G. Bachas, Science, 2004, 303, 62–65 CrossRef CAS PubMed.
- A. Bala Subramaniam, M. Abkarian, L. Mahadevan and H. A. Stone, Nature, 2005, 438, 930 CrossRef CAS PubMed.
- T. A. Hilder, D. Gordon and S.-H. Chung, Small, 2009, 5, 2183–2190 CrossRef CAS PubMed.
- T. A. Hilder, R. Yang, D. Gordon, A. P. Rendell and S.-H. Chung, J. Phys. Chem. C, 2012, 116, 4465–4470 CAS.
- X. Gong, J. Li, K. Xu, J. Wang and H. Yang, J. Am. Chem. Soc., 2010, 132, 1873–1877 CrossRef CAS PubMed.
- J. Su and H. Guo, J. Phys. Chem. B, 2012, 116, 5925–5932 CrossRef CAS PubMed.
- J. C. Phillips, R. Braun, W. Wang, J. Gumbart, E. Tajkhorshid, E. Villa, C. Chipot, R. D. Skeel, L. Kalé and K. Schulten, J. Comput. Chem., 2005, 26, 1781–1802 CrossRef CAS PubMed.
- W. L. Jorgensen, J. Chandrasekhar, J. D. Madura, R. W. Impey and M. L. Klein, J. Chem. Phys., 1983, 79, 926–935 CrossRef CAS PubMed.
- A. D. MacKerell, D. Bashford, M. Bellott, R. L. Dunbrack, J. D. Evanseck, M. J. Field, S. Fischer, J. Gao, H. Guo, S. Ha, D. Joseph-McCarthy, L. Kuchnir, K. Kuczera, F. T. K. Lau, C. Mattos, S. Michnick, T. Ngo, D. T. Nguyen, B. Prodhom, W. E. Reiher, B. Roux, M. Schlenkrich, J. C. Smith, R. Stote, J. Straub, M. Watanabe, J. Wiórkiewicz-Kuczera, D. Yin and M. Karplus, J. Phys. Chem. B, 1998, 102, 3586–3616 CrossRef CAS PubMed.
- I. S. Joung and T. E. Cheatham, J. Phys. Chem. B, 2008, 112, 9020–9041 CrossRef CAS PubMed.
- R. Kjellander and H. Greberg, J. Electroanal. Chem., 1998, 450, 233–251 CrossRef CAS.
- S. Kumar, P. W. Payne and M. Vásquez, J. Comput. Chem., 1996, 17, 1269–1275 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |