Novel carbon nitride composites with improved visible light absorption synthesized in ZnCl2-based salt melts†
Abstract
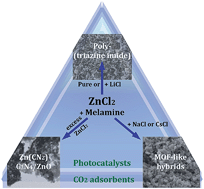
Poly(triazine imide)-based carbon nitride materials with BET surface areas up to 200 m2 g−1 were synthesized in ZnCl2 containing salt melts without the use of hard templates. We found that the composition, structural order, optical properties and morphology of the products can be adjusted by careful selection of synthesis parameters. The nature of the salt eutectic and precursor concentration in the melt have an especially large influence, with ZnCl2 being a reactive solvent. This novel synthesis route provides access to easily processable materials with improved optical absorption in the visible range that can be used as composite photocatalysts, CO2 adsorbents or nanocomposite fillers.

 Please wait while we load your content...
Please wait while we load your content...