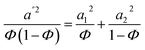DOI:
10.1039/C4RA08220F
(Paper)
RSC Adv., 2014,
4, 55341-55348
Electromagnetic shielding materials and coatings derived from gelation of multiwall carbon nanotubes in an LCST mixture
Received
6th August 2014
, Accepted 7th October 2014
First published on 7th October 2014
Abstract
Thermally induced demixing in an LCST mixture, polystyrene (PS)/poly[vinyl methyl ether] (PVME), was used as a template to design materials with high electrical conductivity. This was facilitated by gelation of multiwall carbon nanotubes (MWNTs) in a given phase of the blends. The MWNTs were mixed in the miscible blends and the thermodynamic driven demixing further resulted in selective localization in the PVME phase of the blends. This was further confirmed by atomic force microscopy (AFM). The time dependent gelation of MWNTs at shallow quench depth, evaluated using isochronal temperature sweep by rheology, was studied by monitoring the melt electrical conductivity of the samples in situ by an LCR meter coupled to a rheometer. By varying the composition in the mixture, several intricate shapes like gaskets and also coatings capable of attenuating the EM radiation in the microwave frequency can be derived. For instance, the PVME rich mixtures can be molded in the form of a gasket, O-ring and other intricate shapes while the PS rich mixtures can be coated onto an insulating polymer to enhance the shielding effectiveness (SE) for EM radiation. The SE of the various materials was analyzed using a vector network analyzer in both the X-band (8.2 to 12 GHz) and the Ku-band (12 to 18 GHz) frequency. The improved SE upon gelation of MWNTs in the demixed blends is well evident by comparing the SE before and after demixing. A reflection loss of −35 dB was observed in the blends with 2 wt% MWNTs. Further, by coating a layer of ca. 0.15 mm of PS/PVME/MWNT, a SE of −15 dB at 18 GHz could be obtained.
Introduction
The drastic increase in the usage of electronic equipment of late has led to a very serious problem. The electromagnetic waves generated from this equipment interfere with the precise electronics of the surrounding devices and more importantly cause serious health hazards. This is often referred to as electromagnetic pollution and hence, the urgent need of the current era is to shield this EM radiation. A good shielding material must be capable to shield both incoming and outgoing radiation.1 The current generated by the electromagnetic shield creates an electronic charge of different polarity along the shield. Any joints or gaps present in the shield can cause the current to run from the surface to inside causing a break in the shield and thereby reducing the shielding effectiveness (SE). A proper electronic design suggests different techniques of shielding, like enclosure, gasketing, filtering, grounding, etc. to avoid this reduction in shielding. The efficiency of shielding is dependent on the dielectric and magnetic properties of the material. The principle of the present shielding technology stems from the concept of a ‘Faraday cage’, where a sensitive instrument can be encapsulated in a thin, conducting material. The alternating magnetic field in a conducting material can induce eddy currents which induce a magnetic field of opposite orientation inside the shield.1
Due to high electrical conductivity, metals are currently being used as shielding materials. However, the susceptibility of metals to corrosion is a major drawback of their widespread use in commercial applications. In this context, due to the improved flexibility, design freedom and corrosion resistance, conducting polymeric composites have emerged as an economic alternative to conventional materials for Electromagnetic interference (EMI) shielding applications. Polymers are inherently non-conducting and are transparent to EM waves. These materials can be made into an efficient shielding material either by blending with conducting nanoparticles or by using intrinsically conducting polymers. The polymeric nanocomposites shield via a combination of reflection and absorption, unlike reflection which is dominant in the case of metals. The intrinsically conducting polymers have certain disadvantages over conducting polymer composites with regards to their poor thermal and mechanical stability. Hence, polymeric nanocomposites with conducting particles like carbon black, carbon nanofibers (CNF), carbon nanotubes (CNTs), etc. have been developed, which are capable of providing efficient SE.2,3
The total shielding effectiveness varies with the thickness of the sample, frequency of the measurement, processing of the material etc. In the recent past, multiwall carbon nanotubes (MWNTs) have been widely used in various polymeric materials like PS, PMMA, PP, PU, etc. to develop lightweight EMI shielding materials.4–9 Conductivity of the order of ca. 10−4 S cm−1 and SE of ca. −20 dB has been achieved in these materials however at relatively higher loadings of MWNTs. In this context, selective localization of MWNTs in a bi-continuous blend can increase the local concentration of the nanoparticles, thereby paving the way for interconnected structures.10 High electrical conductivity is one of the key requirements for SE because when an electric field is applied to the surface of an ideal conductor, the current that is induced results in charge displacement, thereby cancelling the applied field.11,12 A low percolation threshold has been reported by selectively localizing conducting particles in one of the phases of a co-continuous blend.13
In the case of lower critical solution temperature (LCST) blends, demixing can lead to different transient microstructures. The latter can be trapped at different length scales by selective filling of nanoparticles. In the case of PS/PVME, dynamic asymmetry14,15 leads to different phase separation mechanisms like spinodal decomposition, viscoelastic phase separation etc. which in turn result in different microstructures. In our earlier study, we have systematically investigated the effect of MWNTs on the segmental dynamics and intermolecular cooperativity in PS/PVME blends.16–19 In this study, two different compositions of PS/PVME were assessed with respect to shielding efficiency as a function of MWNT content. One of the unique advantages of these materials is that they can be molded into various shapes unlike PS which is very brittle and PVME which is very tacky. In addition, depending on the composition, they can either be shaped into dough or other intricate shapes like gaskets, O-rings etc. As PVME has both hydrophilic and hydrophobic groups, they are excellent materials for coating on various thermoplastics including hydrophobic olefins.20 In light of this, we have chosen different compositions to design materials and coatings to attenuate EM radiations. Thermally induced demixing in PS/PVME blends has been employed to organize MWNTs in the blends to design materials with high electrical conductivity and shielding effectiveness.
Materials and sample preparation
Atactic polystyrene (PS) with Mw of 35 kDa and PDI of 2.0 was procured from Sigma-Aldrich (USA). Poly[vinyl (methyl ether)] (PVME) of 80 kDa was obtained from Tokyo Chemical Industry Co., Ltd (Japan) and was obtained as a 30% solution in water. Analytical grade solvents were obtained commercially and used as received. The 50/50 and 70/30 (wt/wt) PS/PVME blends were prepared by shear mixing (Ultra-Turrax® T25) in toluene at 8000 rpm for 45 min. MWNTs were initially dispersed in toluene using a probe sonicator (Heilscher UP 400S). The composite solution was then dried under vacuum at room temperature for two days and at 60 °C for two days. Polymer solutions of 2 wt% in toluene were prepared for atomic force microscopy (AFM). The samples were spin coated on a Si substrate at 3000 rpm for 2 min. This was annealed at 115 °C for one hour. The evolved morphology was arrested by quenching the sample in liquid nitrogen.
Characterization
Phase separated morphology and localization of MWNTs upon demixing in the annealed blends was evaluated using Dimension Icon ScanAsyst™ AFM. The phase morphology was probed by using a soft tapping mode. Conductivity measurements were done using an electro-rheological set-up wherein a DHR-3 rheometer from TA Instruments was coupled to a LCR meter (Agilent E4980A) at a constant frequency 20 Hz at 1 V. In order to study the electromagnetic interference SE in X and Ku-band frequency range an Anritsu MS4642A vector network analyzer (VNA) was coupled with a coax (Damaskos M07T). The instrument was calibrated by SOLT (Short-Open-Load and Reference Through) for full two ports to avoid absorption and reflection losses due to the transmission line and sample holder. Toroidal shaped samples were compression molded in the case of PS/PVME 70/30 composition and was molded by hand in the case of PS/PVME 50/50 composition. S parameters (S11, S12, S22 and S21) were measured in the X-band and Ku-band frequency regions. Further, 100 mg of the sample in toluene (20 vol%) was prepared and was dip coated onto an insulating PVDF sample followed by drying for two days before measuring the scattering parameter. Toluene is a non-solvent for PVDF and hence we do not expect PVDF to swell in toluene. The thickness of the coating was estimated to be ca. 0.15 mm.
Results and discussion
Phase morphology and localization of MWNTs in the demixed blends
One of the major advantages of using a PS/PVME blend is their flexibility and the ease of molding into various intricate shapes like gaskets, O-rings etc. which can be further explored as EMI shielding materials, coatings, sealants etc. Some of the intricate shapes are shown in Fig. 1. These shapes can be molded without any post processing techniques and can be easily molded into any volumetric shapes. The fabrication can be done at room temperature and also close to the Tg of PS (∼70 °C) by using a dough.
 |
| | Fig. 1 Intricate shapes molded using various PS/PVME compositions. | |
As mentioned earlier, MWNTs were mixed in the homogeneous blends. Phase separation in the blends is studied by rheology.21,22 We have traced the rheological phase separation temperature by performing isochronal temperature sweeps. It is well reported that rheology takes into account the global demixing in the blends. The correlation length, which is the length scale of the concentration fluctuation, increases abruptly at the vicinity of the phase separation temperature. ξ is given by,
| |
 | (1a) |
where
χ is the interaction parameter,
χS is the interaction parameter at spinodal temperature (
Ts) and
a′′ is the characteristic length. The individual length segments are given by the equation,
| |
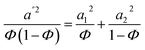 | (1b) |
| |
 | (1c) |
The data obtained from isochronal temperature ramp (storage and loss modulus) measurements can be used to understand the contribution of the correlation length (ξ) of the concentration fluctuation to the evolving stresses and can be derived from the isochronal temperature sweeps (see Fig. 2a). The temperature at which the correlation length increases rapidly can hence be taken as the binodal temperature. The correlation length (ξ−2) is plotted as a function of temperature in Fig. 2b for 70/30 PS/PVME blends with 2 wt% MWNTs as an example.23 A straight line intercepting the X-axis can be taken as the spinodal decomposition temperature.
| |
 | (1d) |
 |
| | Fig. 2 (a) ξ with respect to temperature plot, (b) 1/ξ2 vs. 1/T plot derived from temperature sweep of PS/PVME 70/30 with 1 wt% MWNT. | |
We have chosen a temperature (115 °C) which lies in the intermediate quench depth to systematically study the evolution of electrical conductivity as the blends phase separate. The phase separation temperatures for all the compositions studied are listed in Table 1. The thermodynamics that drive phase separation allows the MWNTs to migrate to their energetically favoured phase during demixing. In order to assess the localization of MWNTs in the blends, the samples were annealed for 1 h at 115 °C and the morphology was scanned using AFM. The difference in the surface tension between PVME (29 mN m−1) and PS (36 mN m−1) causes various domains in this laterally phase separating blend. The MWNT are observed in the energetically favoured phase i.e. PVME which is also more polar than PS even though the solubility parameter of PVME (δ = 22.6 MPa1/2) is comparable to that of PS (δ = 19.2 MPa1/2).24 Fig. 3 shows the 2D phase and 3D height images of phase separated blends of PS/PVME 70/30 with 1% MWNTs. Due to low interfacial tension and preferential affinity, PVME wets both the air–polymer interface and silicon substrate-polymer interface and leads to surface enrichment. This was explained in more detail in our previous work25 by using XPS.26–28 The localization of MWNTs can be better understood in the 3D phase image. It appears that annealing for 1 h has resulted in coarser morphology but the MWNTs in the PVME phase can be well discerned from the AFM micrographs.
Table 1 Phase separation temperatures for various compositions
| Composition PS/PVME (wt/wt) + wt% MWNT |
Phase separation temperature |
| Onset (°C) |
Spinodal (°C) |
| 50/50 + 0.5 |
110 |
121 |
| 50/50 + 1.0 |
90 |
118 |
| 70/30 + 0.5 |
98 |
118 |
| 70/30 + 1.0 |
110 |
119 |
| 70/30 + 2.0 |
108 |
132 |
 |
| | Fig. 3 AFM images of 70/30 PS/PVME blends with 1 wt% MWNTs (a) 2D phase image, (b) 3D phase image (1 μm). | |
Evolution of electrical conductivity using electro-rheology set-up
One of the rationales of the paper was to monitor the evolution of electrical conductivity at an intermediate quench depth. We employed an electro-rheology set-up to gain insight into the effect of different transient microstructures on the electrical conductivity of the demixed samples.4 As we determined the phase separation temperature rheologically, monitoring the evolution of electrical conductivity by an electro-rheology set-up would be more appropriate as different techniques can yield different demixing temperature. The samples used in both rheological experiments and electro-rheology are identical and hence we believe that the temperature chosen is in the intermediate quench depth which was obtained from the rheological experiments.
From AFM it was well evident that MWNTs migrated to their preferred phase driven by thermally induced demixing. Apart from increasing the local concentration in a given phase, it also manipulates the phase separation dynamics. The former leads to a dramatic increase in the electrical conductivity in the blends.13,29,30 In the case of PS/PVME blends, the segmental relaxations can be coupled with the conductivity.17 As described earlier, the evolution of electrical conductivity was monitored by a LCR meter coupled to a rheometer (see the schematic representation in Fig. 4a). Fig. 4b and c depicts the time dependent variation in conductivity in the blends investigated here. It is important to note that the samples were loaded onto the geometry at 70 °C, which is well below the rheological demixing temperature. However, it takes about 3 min to reach 115 °C during which we expect the microstructure to evolve, though not significantly. Hence, the initial conductivity cannot be taken as a real measure as the morphology would have evolved by then. However, our objective was to monitor the evolution of electrical conductivity at an intermediate quench depth, where the evolution can be studied at least in a relevant experimental time scale. The variation in conductivity is monitored for an hour under isothermal condition (115 °C). For all composites of 70/30 blends studied here, there was an increase of about an order of magnitude in the conductivity upon annealing the blends, whereas the composites of 50/50 composition showed a less prominent increase in conductivity. The compositional difference will cause a difference in the effective concentration of MWNT in the PVME phase. The PS rich blends, i.e. 70/30 PS/PVME, has a higher effective concentration of MWNT in the PVME phase and it also manifests in higher electrical conductivity. This observation scales with increasing concentration of MWNTs in the blends. As the temperature increases, the mobility of the polymer chains increases due to Brownian motion. In the intermediate quench depth, a dominant thermodynamic force is generated due to the formation of dynamic PS domains.31 The studies of Sumita13 and Wessling32 reported that the dispersion of fillers did not lead to a thermodynamic equilibrium. The percolation threshold achieved was hence due to the interfacial interaction of the particles to the component associated with the phase separation process. The improved conductivity on annealing is due to the increased MWNT–MWNT interaction.33
 |
| | Fig. 4 (a) Schematic of the electro rheology set-up. (b), (c) AC conductivity as a function of time at 115 °C for various compositions of PS/PVME. | |
Shielding effectiveness
Shielding effectiveness is defined as the ability of a material to attenuate EM radiation. It is expressed as the ratio of the incoming power to the outgoing power of electromagnetic radiation.| |
 | (2a) |
where, Pt and Pi are the transmitted and incident power (in watts), respectively. The total electromagnetic shielding effectiveness of a material (SET) is the sum of reflection, absorbance and multiple reflection losses. It is expressed as:| | |
SET(dB) = SER + SEA + SEI
| (2b) |
where SER is the shielding effectiveness due to reflection, SEA is the shielding effectiveness due to absorption and SEI is the shielding effectiveness due to multiple reflections. Multiple reflections in a material are caused due to the internal reflections within the material. This is valid only in the case of thin samples, where the sample thickness is less than the skin depth. The VNA and the coax set-up used in this study is shown in Fig. 5a. The SE depends on the permeability and the permittivity of the material. High real permittivity and dielectric loss in a material contribute to the improved SE.34 Reflection, which is considered as the primary mechanism for shielding in polymeric nanocomposites, is caused due to the mobile charge carriers like electrons and holes. The absorption losses are enhanced by the presence of materials with electric or magnetic dipoles which are capable of interacting with the electromagnetic waves.35 The current and the voltage travelled through the transmission line scatter because of the differences in impedance of the air and varying discontinuity in the material. SE can be evaluated based on the scattering parameters using the following equations:| |
 | (3) |
| | |
SER = 10 × log10(1/(1 − S211))
| (4) |
| | |
SEA = 10 × log10((1 − S211)/S212)
| (5) |
where Sij is the power transmitted from port i to j and S11, S12 and S21 are the forward reflection coefficient, reverse transmission coefficient and forward transmission coefficient, respectively.36,37
 |
| | Fig. 5 (a) Vector network analyzer with coax set-up; SE (b) 50/50 PS/PVME with MWNTs, 70/30 PS/PVME with (c) 1 wt% MWNTs, (d) 2 wt% MWNTs before and after annealing. | |
It was observed that the conductivities of the 70/30 PS/PVME blends were higher than that of the 50/50 blends with MWNTs. The total SE measurements also showed a similar observation in accordance with the conductivity data. The total SE in the case of 50/50 PS/PVME blends with 0.5 wt% and 1 wt% of MWNTs (Fig. 5b) is almost unaltered upon annealing, whereas, a significant change was observed in the case of 70/30 PS/PVME blends, (Fig. 5c and d). 50/50 PS/PVME blends showed slightly enhanced conductivity (less than one order change) compared to PS/PVME 70/30 samples on annealing. It is envisaged that upon demixing, MWNTs migrate to their preferred phase thereby paving the way for their interconnected network like structure in a given phase. This increases the electrical conductivity due to the micro capacitance generated between the adjacent MWNTs.38 The total SE values of various blend compositions analyzed are given in Table 2.
Table 2 Total shielding effectiveness of various compositions
| Composition PS/PVME (wt/wt) + wt% MWNT |
SE dB at 18 GHz |
| Samples as such |
Samples annealed |
| 50/50 + 0.5 |
−9 |
−9 |
| 50/50 + 1.0 |
−15 |
−15 |
| 70/30 + 0.5 |
−12 |
−15 |
| 70/30 + 1.0 |
−17 |
−24 |
| 70/30 + 2.0 |
−24 |
−28 |
Thorough information on the absorption losses of the material is obtained by evaluating the variation in reflection losses of a material as a function of frequency. The reflection loss is estimated from the complex permeability and permittivity by applying the transmission line theory.
| |
 | (6) |
| |
 | (7) |
where
Zin is the input impedance of the absorber,
f is the frequency,
d is the thickness of the sample and
c is the velocity of light.
Fig. 6 gives the reflection loss as a function frequency for different blends investigated here. The dip in the RL plot indicates minimal reflection of electromagnetic radiation.
39 50/50 PS/PVME blends with MWNTs showed a maximum of −20 dB at 0.5 wt% MWNTs and no significant change was observed upon increasing the fraction of MWNTs in the blends. However, 70/30 PS/PVME blends with 1 wt% MWNT showed a maximum reflection loss of −27 dB at 13 GHz and the samples with 2% MWNT showed a maximum reflection loss of −31 dB at 16 GHz.
 |
| | Fig. 6 Reflection loss as a function of frequency for PS/PVME blends with MWNTs (a) 50/50 and (b) 70/30. | |
PS/PVME blends as EMI coatings
PVME is an excellent material used for coating and adhesive applications. The surface tension of PVME is 27 mJ m−2, which is comparable to that of various other polymers like polypropylene (PP) [29 mJ m−2], polyethylene (PE) [31 mJ m−2], and polyvinylidene fluoride (PVDF) [25 mJ m−2]. This suggests that PVME is capable of wetting these polymers with less heat of mixing. The comparable solubility parameters in the range 8.5 to 12.7 (cal/cm–3)1/2 also make PVME an excellent material for coating these thermoplastics.40 The blend of PVME and many styrene copolymers has been used for coating metal foils, plastics and so on. The coatings made of PS/PVME blends with MWNTs, which showed significant conductivity and sufficient thickness, are capable of attenuating EM radiation. The coating process is schematically represented in Fig. 7. PS/PVME 70/30 blend with 2 wt% MWNTs was made a solution in toluene 10 wt/vol and was coated onto a non-conducting homopolymer substrate. PVDF was chosen as the substrate due to the ease of processability and availability. This composition was ideal in preparing coatings as it is capable of making the coating adhere on the PVDF surface without showing any tacky nature, which is seen in blends with high concentrations of PVME. The PS/PVME coatings of thickness ca. 0.15 mm gave shielding efficiency of −15 dB on non-shielding PVDF substrate (Fig. 8). This suggests the prospect of the PS/PVME blend forming a highly shield efficient coating material. The same composition of the blend which formed the stable coating on PVDF had also been compression molded to obtain quite a stiff donut shaped sample used for the EMI shielding measurements.
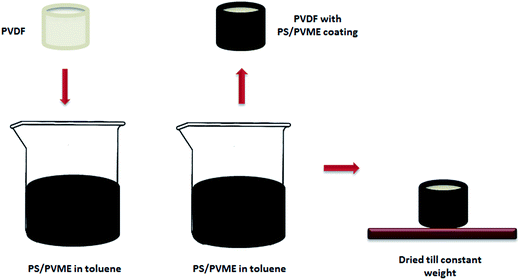 |
| | Fig. 7 Schematic representation showing the procedure for dip coating a PVDF sample with PS/PVME/MWNT. | |
 |
| | Fig. 8 Total SE as a function of frequency for PS/PVME 70/30 with 2.0 wt% MWNTs coated onto a PVDF sample. | |
As mentioned earlier, these blends can be molded into various intricate shapes like gaskets, O-rings, etc. PS/PVME 50/50 blends were suitable for such applications. High concentration of PS (i.e. more than 70%) in the samples makes them stiffer and brittle, hence making them incapable of being molded into various shapes at room temperature. PS/PVME 50/50 blends can also be coated on to PVDF, but owing to the high conductivity obtained in the PS/PVME 70/30 compositions, all the coating measurements were restricted to PS/PVME 70/30 compositions.
Conclusions
Thermally induced demixing in PS/PVME blends with MWNTs was used as a tool to design materials with high electrical conductivity and EMI SE. A range of various shapes and forms were developed by varying the composition in the blends. The time dependent gelation of MWNTs in the metastable regions was studied by monitoring the melt electrical conductivity of the sample in situ by a LCR meter coupled to a rheometer. Various intricate shapes like gaskets and coatings to attenuate the EM radiations in the microwave frequency can be derived by varying the composition in the mixture. For instance, PVME rich blends can be shaped into dough, gaskets, O-rings etc. while PS rich blends can be used as coatings to attenuate EM radiation. 70/30 PS/PVME blends with 1 wt% MWNT showed a maximum reflection loss of −24 dB at 12 GHz and the samples with 2% MWNT showed a maximum reflection loss of −36 dB at 8.65 Hz. Further, a coating of ca. 0.15 mm thick on an EM transparent sample resulted in a shielding efficiency of −15 dB.
Acknowledgements
The authors gratefully acknowledge the Department of Science and Technology, India for the financial support.
References
- X. C. Tong, Advanced materials and design for electromagnetic interference shielding, CRC Press, 2008 Search PubMed.
- M. H. Al-Saleh, W. H. Saadeh and U. Sundararaj, Carbon, 2013, 60, 146–156 CrossRef CAS PubMed.
- M. H. Al-Saleh and U. Sundararaj, Carbon, 2009, 47, 1738–1746 CrossRef CAS PubMed.
- S. P. Pawar, K. Pattabhi and S. Bose, RSC Adv., 2014, 4, 18842–18852 RSC.
- M. Sharma, S. Sharma, J. Abraham, S. Thomas, G. Madras and S. Bose, Mater. Res. Express, 2014, 1, 035003 CrossRef.
- R. Rohini and S. Bose, ACS Appl. Mater. Interfaces, 2014, 6, 11302–11310 CAS.
- Z. Liu, G. Bai, Y. Huang, Y. Ma, F. Du, F. Li, T. Guo and Y. Chen, Carbon, 2007, 45, 821–827 CrossRef CAS PubMed.
- S.-M. Yuen, C.-C. M. Ma, C.-Y. Chuang, K.-C. Yu, S.-Y. Wu, C.-C. Yang and M.-H. Wei, Compos. Sci. Technol., 2008, 68, 963–968 CrossRef CAS PubMed.
- A. A. Koval'chuk, V. G. Shevchenko, A. N. Shchegolikhin, P. M. Nedorezova, A. N. Klyamkina and A. M. Aladyshev, Macromolecules, 2008, 41, 7536–7542 CrossRef.
- S. Bose, A. R. Bhattacharyya, R. A. Khare, A. R. Kulkarni and P. Pötschke, Macromol. Symp., 2008, 263, 11–20 CrossRef CAS.
- D. Chung, J. Mater. Eng. Perform., 2000, 9, 350–354 CrossRef CAS.
- D. Chung, J. Mater. Sci., 2004, 39, 2645–2661 CrossRef CAS.
- M. Sumita, K. Sakata, S. Asai, K. Miyasaka and H. Nakagawa, Polym. Bull., 1991, 25, 265–271 CrossRef CAS.
- R. Larson and G. H. Fredrickson, Macromolecules, 1987, 20, 1897–1900 CrossRef CAS.
- H. Tanaka, Phys. Rev. Lett., 1993, 71, 3158–3161 CrossRef CAS.
- A. Bharati, P. Xavier, G. P. Kar, G. Madras and S. Bose, J. Phys. Chem. B, 2014, 118, 2214–2225 CAS.
- D. Cangialosi, A. Alegría and J. Colmenero, Macromolecules, 2008, 41, 1565–1569 CrossRef CAS.
- S. Zhang and J. Runt, J. Phys. Chem. B, 2004, 108, 6295–6302 CrossRef CAS PubMed.
- G. P. Kar, P. Xavier and S. Bose, Phys. Chem. Chem. Phys., 2014, 16, 17811–17821 RSC.
- A. Kowalski, Z. Czech and Ł. Byczyński, J. Coat. Technol. Res., 2013, 10, 879–885 CrossRef CAS.
- P. J. Flory, Principles of polymer chemistry, Cornell University Press, 1953 Search PubMed.
- A. Ajji and L. Choplin, Macromolecules, 1991, 24, 5221–5223 CrossRef CAS.
- C. C. Han, B. J. Bauer, J. C. Clark, Y. Muroga, Y. Matsushita, M. Okada, Q. Tran-cong, T. Chang and I. C. Sanchez, Polymer, 1988, 29, 2002–2014 CrossRef CAS.
- P. Xavier and S. Bose, J. Phys. Chem. B, 2013, 117, 8633–8646 CrossRef CAS PubMed.
- A. Bharati, P. Xavier, G. P. Kar, G. Madras and S. Bose, J. Phys. Chem. B, 2014, 118, 2214–2225 CAS.
- B. D. Ermi, A. Karim and J. F. Douglas, J. Polym. Sci., Part B: Polym. Phys., 1998, 36, 191–200 CrossRef CAS.
- D. H. K. Pan and W. Prest, J. Appl. Phys., 1985, 58, 2861–2870 CrossRef CAS PubMed.
- H. Tanaka and T. Araki, Chem. Eng. Sci., 2006, 61, 2108–2141 CrossRef CAS PubMed.
- G. Vleminckx, S. Bose, J. Leys, J. Vermant, M. Wübbenhorst, A. A. Abdala, C. Macosko and P. Moldenaers, ACS Appl. Mater. Interfaces, 2011, 3, 3172–3180 CAS.
- P. Xavier and S. Bose, Phys. Chem. Chem. Phys., 2014, 16, 9309–9316 RSC.
- A. Gharachorlou and F. Goharpey, Macromolecules, 2008, 41, 3276–3283 CrossRef CAS.
- B. Wessling, Polym. Eng. Sci., 1991, 31, 1200–1206 CAS.
- C. Zhang, P. Wang, C.-a. Ma, G. Wu and M. Sumita, Polymer, 2006, 47, 466–473 CrossRef CAS PubMed.
- J. S. Im, J. G. Kim and Y.-S. Lee, Carbon, 2009, 47, 2640–2647 CrossRef CAS PubMed.
- S. M. Abbas, A. K. Dixit, R. Chatterjee and T. C. Goel, J. Magn. Magn. Mater., 2007, 309, 20–24 CrossRef CAS PubMed.
- S. Celozzi, G. Lovat and R. Araneo, Electromagnetic shielding, Wiley Online Library, 2008 Search PubMed.
- J. Dřínovský and Z. Kejik, Meas. Sci. Rev., 2009, 9, 109–112 Search PubMed.
- F. Ren, H. Yu, L. Wang, M. Saleem, Z. Tian and P. Ren, RSC Adv., 2014, 4, 14419–14431 RSC.
- M.-S. Cao, W.-L. Song, Z.-L. Hou, B. Wen and J. Yuan, Carbon, 2010, 48, 788–796 CrossRef CAS PubMed.
- M. A. Kulzick, W. R. Pretzer, T. Y. Lynch and P. A. Koning, Poly(vinylalkylether)-containing hot melt adhesives for Polyethllene and polypropylene, US Pat., 5359006, 1994.
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here.  *
*