Sub-ppm H2S sensor based on NASICON and CoCr2−xMnxO4 sensing electrode
Han Zhanga,
Tiegang Zhongb,
Ruize Suna,
Xishuang Liang*a and
Geyu Lu*a
aState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, 2699 Qianjin Street, Changchun 130012, China. E-mail: liangxs@jlu.edu.cn; lugy@jlu.edu.cn; Fax: +86-431-85167808; Tel: +86-431-85168384 Tel: +86-431-85167808
bCollege of Electronics and Information Engineering, Liaoning Technical University, 188 Longwan South Street, Huludao 125105, PR China
First published on 23rd September 2014
Abstract
Solid electrochemical sensors based on sodium super ionic conductor (NASICON) and spinel-type oxide CoCr2−x MnxO4 (x = 0, 1, 1.2, 1.4 and 2) sensing electrode were designed for sub-ppm H2S detection. In comparison with other spinel-type oxides, the sensor using CoCr1.2Mn0.8O4 showed the maximum response of 178 mV for 10 ppm H2S at 250 °C. The sensor displayed good stability for H2S during the testing period. Moreover, the sensor exhibited excellent selectivity toward H2S against the other interference gases, such as SO2, NO2, CH4, CO, C2H4, H2 and NH3. A sensing mechanism related to the mixed potential was proposed for the sensor based on NASICON and oxide electrodes. The effect of sintering temperature had also been investigated.
1. Introduction
Hydrogen sulfide, one of the chemical industry's by-products is strongly harmful, toxic and flammable when liberated into the atmosphere. Therefore, a high performance sensor, which has the characteristics of high sensitivity, low detecting limit, good selectivity and stability, is urgently desired for exactly detecting hydrothion in the atmosphere.1,2 In the past few decades, hydrogen sulfide sensors using metal-oxide semiconductors, such as SnO2–CuO,3–5 Pt-doped α-Fe2O3,6 and noble metal doped WO3,7 have been widely investigated as sensing materials owing to their high response to the target gases and simplicity in synthesis. Though these explorations promoted the development of an H2S sensor and obtained exciting results, developing new H2S sensor strategies are always increasing for better sensitivity, selectivity and stability. Moreover, the detection limits of these semiconductor sensors are at the ppm level, but the acceptable ambient levels of H2S (recommended by the Scientific Advisory Board on Toxic Air Pollutants, USA) are in the range of sub-ppm.8 To the solid electrolyte type gas sensor, it displays the potential for detecting H2S at ppb level by optimizing the corresponding sensitive electrode material. In addition, most research focused on the sensors using yttria stabilized zirconia (YSZ) and sodium super ionic conductor (NASICON). For instance, our recent work reported a sub-ppm H2S sensor based on YSZ and hollow balls NiM2O4 (M = Mn, Cr, Fe) sensing electrode, which showed good sensing properties for H2S at 500 °C.9 However, YSZ-based sensors are generally operated at high temperature (500–700 °C); thus, their power dissipation is high. Compared with sensors based on YSZ, the NASICON-based sensors can be operated at the intermediate temperatures (200–500 °C). Hence, they have lower power consumption and are more suitable for detecting toxic gases in the atmospheric environment.10–12The choice of sensing material, which is a key element of solid electrolyte gas sensor is crucial for the sensor performance. Spinel-type oxides, well known for their wide applications in the CO hydrogenation process,13 catalytic removal of NOx,14 a cathode material of lithium ion battery15 and sensing electrode of the solid electrolyte sensors,16–21 are represented by the chemical formula AB2O4 in which A ions are generally divalent cations occupying tetrahedral sites and B ions are trivalent cations in octahedral sites. In our previous work, CoCr2O4 was utilized as a sensing material to fabricate the mixed potential type Cl2 sensor showing excellent sensing properties.22 In order to explore the spinel oxides with high catalytic activity, CoCrxMn2−xO4 spinel oxides were prepared as the sensing material in the present work.
In this work, H2S sensors using spinel-type oxides CoCr2−xMnxO4 as sensing electrodes were fabricated, and their gas sensing properties were studied. A comparative gas sensing study between the CoCr2O4, CoMn2O4, CoCrMnO4, CoCr1.2Mn0.8O4, CoCr1.4Mn0.6O4 was performed. The sensor using CoCr1.2Mn0.8O4 displayed the highest response to hydrothion among these oxides. The sensor resulted in a response of 27 mV for 100 ppb H2S at 250 °C, which also exhibited good selectivity and stability. The influence of the sintering temperature on sensing the performance of the sensor attached with CoCr1.2Mn0.8O4 electrode was investigated.
2. Experimental section
2.1 Synthesis of the sensing materials
NASICON was prepared from ZrO(NO3)2, NaNO3, (NH4)2HPO4 and Si(C2H5O)4 by the sol–gel process and sintered at 1000 °C for 12 h.23 The spinel-type oxide CoCrxMn2−xO4 (0, 1, 1.2, 1.4, 2) that served as the sensing electrodes were synthesized by the autocombustion of ethylene glycol–metal nitrate polymerized gel precursors. The powders of Co(NO3)2·6H2O, Cr(NO3)2·9H2O, and Mn(NO3)2·4H2O were dissolved in 20 ml deionized water. Then, the concentrated nitric acid of 10 ml was added to the above mixed nitrate solution and the mixed solution was stirred at room temperature. During the stirring process, 80 ml ethylene glycol (EG) was added to the above solution to form a gel. The obtained gel was heat treated at 80 °C for 16 h, followed by drying at 130 °C for 24 h and sintering at 800 °C in air for 3 h.24 In addition, to study the effect of sintering temperature on sensor performance the CoCr1.2Mn0.8O4 was sintered at 600, 800 and 1000 °C in air for 3 h.2.2 Fabrication and measurement of gas sensors
The structure of the sensor is shown in Fig. 1. A thick film of NASICON was formed on the outer surface of the alumina tube and sintered at 900 °C for 6 h. Then, two Au electrodes with ring shapes were formed at the two ends of the NASICON thick film by applying the commercial Au paste and calcining at 800 °C for 20 min. Then, a composite oxide layer (CoCr2O4, CoMn2O4, CoCrMnO4, CoCr1.2Mn0.8, and CoCr1.4Mn0.6O4) as the sensing electrode was formed on one side of the ring shaped Au electrode, followed by sintering at 600 °C for 3 h. The Ni–Cr coil inserted into the alumina tube provides the operating temperature for the sensors. Devices attached with CoCr1.2Mn0.8O4 sintering at 600, 800 and 1000 °C were numbered as S600, S800 and S1000. The gas sensing properties of the sensors were measured by a conventional static method at a constant temperature and humidity environment (26 °C, 30% RH).25,26 The sample gases containing different H2S concentrations were obtained by diluting 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm H2S with air. When the sensor was exposed to clean air or the sample gas, the electric-potential difference (V) was measured with a digital electrometer (Rigol. DM3054) as a sensing signal and the results obtained were registered with a computer connected to the electrometer.
000 ppm H2S with air. When the sensor was exposed to clean air or the sample gas, the electric-potential difference (V) was measured with a digital electrometer (Rigol. DM3054) as a sensing signal and the results obtained were registered with a computer connected to the electrometer.
The XRD patterns of the composite oxides were recorded by Rigaku wide-angle X-ray diffractometer (D/max rA, using Cu Kα radiation at wavelength λ = 0.1541 nm). The complex impedance of the sensor in sample gas was measured using an impedance analyzer (Solartron, 1260 and Solartron, 1287) in the frequency range of 0.1 Hz–1 MHz. The cyclic voltammograms for the sensors were measured by an electrochemical interface and impedance/gain phase analyzer (Solartron SI 1287 and SI 1260).
2.3 Characterization of the sensing materials
Fig. 2(a) shows the XRD patterns of CoCr2O4, CoMn2O4, CoCrMnO4, CoCr1.2Mn0.8, and CoCr1.4Mn0.6O4. Fig. 2(b) shows the XRD patterns of CoCr1.2Mn0.8O4 sintered at 600, 800 and 1000 °C for 3 h. It can be seen that CoCr2O4 and CoMn2O4 retained their crystallographic phases (cubic) corresponding to JCPDS PDFs (#78-711 and #77-471, respectively), and were testified to be spinel-type oxides. Moreover, the as-prepared oxides (CoCrxMn2−xO4) retained their crystallographic phases (cubic) corresponding to JCPDS PDF (#70-2465). With increasing the sintering temperature, the intensity of the peaks of CoCr1.2Mn0.8O4 increased, which suggests that the crystallization quality was improved with increasing sintering temperature. | ||
| Fig. 2 (a) X-ray diffraction (XRD) pattern of different sensing material, (b) X-ray diffraction (XRD) pattern of CoCr1.2Mn0.8O4 sintered at various temperatures. | ||
3. Results and discussion
3.1 Sensing the performances of the sensors
The sensing mechanism of the above mentioned H2S sensor related to the mixed potential. The present sensor can be expressed by the following electrochemical cell:| H2S (+air), Au |NASICON| Au, CoCrxMn2−xO4, H2S (+air) |
When H2S gas molecules arrive at the triple-phase boundary (sensing material-gas-electrolyte), the following cathodic (1) and anodic (2) reactions simultaneously occurred at the TPB:
| H2S + O2 + Na2O (NASICON) → 2Na+ + SO2 + H2O + 2e− | (1) |
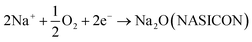 | (2) |
When the rates of the above chemical reactions were equal to each other they arrived at a dynamic equilibrium, and the electrode potential at the sensing electrode was regarded as the mixed potential.27 To these semireactions, either promoting semireaction (1) or restraining semireaction (2) will improve the potential of SE.
In order to find out the most suitable sensing material among synthetic oxides, the comparison of gas sensing study between the CoCr2O4, CoMn2O4, CoCrMnO4, CoCr1.2Mn0.8O4, CoCr1.4Mn0.6O4 was carried out. The responses (VH2S – Vair) to 10 ppm H2S for the sensors attached with different oxides (CoCr2O4, CoMn2O4, CoCrMnO4, CoCr1.2Mn0.8O4, CoCr1.4Mn0.6O4) sensing electrodes were tested and are shown in Fig. 3. Among these oxides examined, CoCr1.2Mn0.8O4 showed the highest response to 10 ppm H2S.
For rationalizing the abovementioned results, the polarization curves were obtained in air and in the sample gas (10 ppm H2S + air) at 250 °C using the method previously reported.28 Fig. 4 shows the modified polarization curves for the sensors attached with different sensing materials (CoCr2O4, CoMn2O4, CoCrMnO4, CoCr1.2Mn0.8O4 and CoCr1.4Mn0.6O4). The polarization curves in air are apparently related to the cathodic reaction (2), and in H2S are for the anodic reaction (1). As shown in Fig. 11, on one hand, the slope of the polarization curve for the CoCr1.2Mn0.8O4-attached sensor in air was obviously lower than those polarization curves of other devices, which means that the catalytic activity of CoCr1.2Mn0.8O4 to O2 was the lowest among these sensing materials. On the other hand, compared with other sensors, the polarization curve slope of the sensor attached with CoCr1.2Mn0.8O4-SE was the biggest to H2S, which means that CoCr1.2Mn0.8O4 to H2S has the highest catalytic activity to H2S. Therefore, the sensor attached with CoCr1.2Mn0.8O4 showed the increased catalytic activity for electrochemical reaction (1) and decreased catalytic activity for reaction (2), resulting in the largest potential value. Thus, CoCr1.2Mn0.8O4 was chosen as the sensing electrode material. The sensing properties of the sensor attached with the CoCr1.2Mn0.8O4 electrode defined as SE material were further investigated in detail as follows. Additionally, the intersecting-potential values (CoCr2O4: 80 mV, CoMn2O4: 100 mV, CoCrMnO4: 160 mV, CoCr1.2Mn0.8O4: 180 mV and CoCr1.4Mn0.6O4: 130 mV) are very similar to those obtained (CoCr2O4: 81 mV, CoMn2O4: 100 mV, CoCrMnO4: 160 mV, CoCr1.2Mn0.8O4: 179 mV and CoCr1.4Mn0.6O4: 127 mV). This coincidence supports the H2S sensing mechanism involving a mixed potential.29
To this kind of gas sensor, the property of the sensing electrode material is significantly influenced by the sintering temperature. Fig. 5 shows the relationship between the sintering temperature and the responses of the sensors (S600, S800 and S1000) for 10 ppm H2S at 250 °C. It was found that the sensor using CoCr1.2Mn0.8O4 sintered at 800 °C had the largest response to 10 ppm H2S at 250 °C.
 | ||
| Fig. 5 Response to 10 ppm H2S for the sensor attached with CoCr1.2Mn0.8O4 sintered at different temperatures. | ||
In order to examine the electrocatalytic properties of the sensing materials sintering at different temperature, Fig. 6 shows a comparison of the cyclic voltammograms for S600, S800 and S1000 cyclic scanned between −5 and 5 V with a scan rate of 10 mV s−1, respectively, in the same concentration (10 ppm) H2S. Compared with the S600 and S1000, S800 obviously exhibited higher peak current for the electrocatalytic oxidation of H2S. At the peak point, the electrochemical redox reactions occurred. Moreover, a large number of electrons were lost and gained on the surface of the electrode, which formed a large value of current. Under the same conditions, the higher peak current means a higher electrochemical rate of reaction. Such a result reveals that the S800 possesses good catalytic activity toward the electrochemical reduction of H2S.
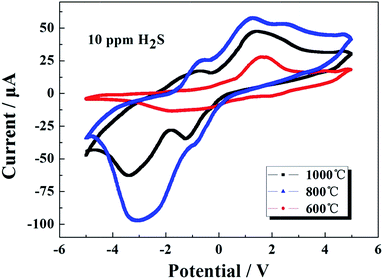 | ||
| Fig. 6 Cyclic voltammograms of sensors attached with CoCr1.2Mn0.8O4 sintering at different temperatures. | ||
Triple-phase boundary (TPB) is a reaction field for the reactions (1) and (2). It is a one-dimensional three-phase (sensing material-gas-electrolyte) coexisting region, which is measured by the three-phase boundary length. A greater three-phase boundary length means more reactive sites. Thus, the microstructure of TPB directly affects the rates of the abovementioned electrochemical reactions. As previously reported,30 the total resistance of the sensor appears to be affected by the interfacial resistance between the SE and NASICON, which is affected by several variables including but not limited to the SE/NASICON interfacial reaction area (namely, the number of reaction sites on TPB). Nyquist plots measured in the sample gas (10 ppm H2S + air) at 250 °C for sensors S600, S800 and S100 are shown in Fig. 7. In the low-frequency range, the interfacial resistance changed with the calcination temperature (R1000 > R800 > R600). With the increase in the sintering temperature, the oxide crystal grain grew and reunited. Therefore, the interface of the particles of SE material and the NASICON increased as the particles enlarged, but the triple-phase boundary reduces as shown in Fig. 8. In this process, the microstructure should be taken into account: the enlarged crystal size reduced the area of the triple-phase boundary, and the reduced area resulted in a decrease in the response value. As described above, the crystallization of the CoCr1.2Mn0.8O4 increased with the elevated temperatures, which affected the electrochemical activity. Therefore, with the increase in the calcined temperature, the crystalline quality and the microstructure of the SE material changed, and both of them affect the variation of the sensor response to H2S. Thus, the best balance between TPB length and electrochemical catalytic activities for CoCr1.2Mn0.8O4 sintered at 800 °C could be formed and generated the largest response to H2S. Thus, the sensing properties of the sensor attached with CoCr1.2Mn0.8O4 sintered at the 800 °C electrode have been further investigated in detail.
Operating temperature as an important parameter of sensor performance was investigated in this work. Fig. 9 displays sensitivity curves of 5–20 ppm H2S for the S800 as sensing electrode at 200 °C, 225 °C, 250 °C, 275 °C and 300 °C, respectively. It could be observed that the highest sensitivity (slope) for 5–20 ppm H2S was obtained at 250 °C. The response for this type of sensor is mainly influenced by the following two factors: 1. the concentration loss, which generated from the oxidation reaction in the oxide layer, affected the sensitivity of the sensor in a negative direction; 2. the rate of the electrochemical reaction at the triple-phase boundary (TPB) affected the sensitivity in the opposite direction. These two factors are related to the chemical and electrochemical catalytic activities of the oxides, respectively. With the increase in the operating temperature, both the chemical and electrochemical activities increases, and the response value of the sensor is decided by the competition of the two processes. At 250 °C, the highest sensitivity was obtained because of the best balance between the H2S molecular diffusion and the electrochemical reaction for the sensor attached with CoCr1.2Mn0.8O4. The sensitivity (slope) was 75 mV/decade. Moreover, the low detection limit of the sensor was 100 ppb. The inset in Fig. 10 exhibits the response-recovery transient for 100 ppb H2S. The response and recovery time of the sensor were less than 100 seconds to 100 ppb H2S.31
 | ||
| Fig. 10 Dependence of ΔV on the H2S concentration and the transients to 0.1 ppm H2S (inset) for the sensor attached with CoCr1.2Mn0.8O4. | ||
Fig. 10 shows the dependence of ΔV on the H2S concentration.
In addition, the continuous response-recovery transients of the sensor for 1 ppm H2S was examined as shown in Fig. 11. Considering the interference of the environment and error in the experiment process, the result indicates that the sensor has good repeatability.
For testifying the long-term stability of the present sensor, the stability of the sensor was investigated by measuring the sensing signal in a specific interval (2 days). The measuring result is displayed in Fig. 12. It could be seen that the sensing signal was very stable during the testing period (20 days), which indicated that the sensor using the CoCr1.2Mn0.8O4 electrode had good stability. It could be because the property of the electrolyte and sensing material tended to be stable after high temperature sintering.
Fig. 13 shows the cross sensitivities of the sensor to H2S and some interference gases at 250 °C. It can be seen that the responses of the sensor for H2S was considerably larger than it was to other gases, such as SO2, NO2, CH4, C2H4, H2, CO and NH3 at 250 °C. Such results indicated that the sensor had excellent selectivity for H2S and showed a potential possibility in practical application.
3.2 Sensing mechanisms of the sensors
If a single electrochemical reaction occurs on an electrode, the electrode potential is known as equilibrium potential. The difference in chemical potential of conducting ions for solid electrolyte between sensing and reference electrodes generates the electromotive force (V), which can be expressed by the Nernstian equation. However, if two or more electrochemical reactions simultaneously take place on the same electrode, the electrode potential is determined by the electrochemical reaction rate involved and is non-Nernstian.Miura et al. have established a sensing mechanism involving mixed potentials for a group of sensors combining stabilized zirconia with oxide electrodes, such as NOx, H2 and CO sensors;32–34 therefore, a similar sensing mechanism would be attempted to apply to the present sensors. In addition, we would quantitatively deal with the local cell.
The electric current densities of the electrochemical oxidation and reduction reactions (1) and (2) can be represented by the following equations:
 | (3) |
 | (4) |
| iH2S + iO2 = 0 | (5) |
We supposed the exchange current densities to obey the following kinetic equations, respectively:
 | (6) |
 | (7) |
| EM = E0 − mAlnCO2 + nAlnCH2S | (8) |
 | (9) |
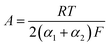 | (10) |
| EM = E0 + mAlnCH2S | (11) |
such analysis can explain the correlation of the EM and H2S concentration shown in Fig. 7.
4. Conclusions
The present paper reported a compact solid electrochemical H2S sensor based on NASICON and composite oxide sensing electrodes. CoCr2−xMnxO4 with spinel structure was prepared by the sol–gel process. The CoCr1.2Mn0.8O4 sintered at 800 °C was confirmed to be the best suited for the sensing electrode. The device attached with CoCr1.2Mn0.8O4 showed excellent sensing characteristics for 0.1–20 ppm of H2S in air at 250 °C. The 90% response and recovery times to 100 ppb H2S at 250 °C were all less than 100 s for the CoCr1.2Mn0.8O4-attached device. The ΔV value of the device was almost linear to the logarithm of H2S concentration, and the slope between ΔV and the logarithm of H2S concentration was 75 mV/decade. The sensor exhibited good stability during the testing period. Moreover, the sensor exhibited excellent selectivity toward H2S against the other interference gases, such as SO2, NO2, CH4, CO, C2H4, H2 and NH3. The sensing mechanism related to the mixed potential could explain the sensing behaviour of the sensor. Therefore, the sensor exhibits great applicable value for detecting hydrothion in the atmosphere based on its excellent sensing performances.Acknowledgements
Supported by NSFC (no. 61104203, 61374218, 61134010, 61327804, 61240014). Program for Changjiang Scholars and Innovative Research Team in University (no. IRT13018) and “863” High Technology Project (2013AA030902) is gratefully acknowledged.Notes and references
- C. M. Ghimbeu, M. Lumbreras, M. Siadat, R. C. Landschoot and J. Schoonman, Sens. Actuators, B, 2008, 133, 694–698 CrossRef CAS PubMed.
- N. S. Ramgir and S. Kailasa, Sens. Actuators, B, 2010, 151, 90–96 CrossRef CAS PubMed.
- M. K. Verma and V. Gupta, Sens. Actuators, B, 2012, 166–167, 378–385 CrossRef CAS PubMed.
- V. Kumar, S. Sen, K. P. Muthe, N. K. Gaur, S. K. Gupta and J. V. Yakhmi, Sens. Actuators, B, 2009, 138, 587–590 CrossRef CAS PubMed.
- J. Tamaki, K. Shimanoe, Y. Yamada, Y. Yamamoto, N. Miura and N. Yamazoe, Sens. Actuators, B, 1998, 49, 121–125 CrossRef CAS.
- Y. Wang, S. Wang, Y. Zhao, B. Zhu, F. Kong, D. Wang, S. Wu, W. Huang and S. Zhang, Sens. Actuators, B, 2007, 125, 79–84 CrossRef CAS PubMed.
- W. H. Tao and C. H. Tsai, Sens. Actuators, B, 2002, 81, 237–247 CrossRef CAS.
- Summary of Rule Amending the Hydrogen Sulfide Acceptable Ambient Level, http://daq.state.nc.us/toxics/studies/H2S/, March 15, 2006.
- Y. Guan, C. Yin, X. Cheng, X. Liang, Q. Diao, H. Zhang and G. Lu, Sens. Actuators, B, 2014, 193, 501–508 CrossRef CAS PubMed.
- X. Liang, T. Zhong, B. Quan, B. Wang and H. Guan, Sens. Actuators, B, 2008, 134, 25–30 CrossRef CAS PubMed.
- Y. Yang and C. Liu, Sens. Actuators, B, 2000, 62, 30–34 CrossRef CAS.
- N. Miura, M. Iio, G. Lu and N. Yamazoe, Sens. Actuators, B, 1996, 35–36, 124–129 CrossRef.
- Q. Liang, K. Chen, W. Hou and Q. Yan, Appl. Catal., A, 1998, 166, 191–199 CrossRef CAS.
- D. Fino, N. Russo, G. Saracco and V. Specchia, J. Catal., 2006, 242, 38–47 CrossRef CAS PubMed.
- M. Hibino, M. Nakamura, Y. Kamitaka, N. Ozawa and T. Yao, Solid State Ionics, 2006, 177, 2653–2656 CrossRef CAS PubMed.
- S. Zhuiykov, T. Ono, N. Yamazoe and N. Miura, Solid State Ionics, 2002, 152–153, 222–229 Search PubMed.
- N. Miura, S. Zhuiykov, T. Ono, M. Hasei and N. Yamazoe, Sens. Actuators, B, 2002, 81, 222–229 CrossRef.
- D. Tsiulyanu, S. Marian, V. Miron and H.-D. Liess, Sens. Actuators, B, 1998, 52, 169–178 CrossRef.
- W. Xiong and G. M. Kale, Sens. Actuators, B, 2006, 114, 101–108 CrossRef CAS PubMed.
- W. J. Moon, J. H. Yu and G. M. Choi, Sens. Actuators, B, 2001, 80, 20–27 CrossRef.
- D. Goran and T. Marija, Sens. Actuators, B, 1989, 18(3/4), 407–414 Search PubMed.
- H. Zhang, X. Cheng, R. Sun, Y. Guan, Y. Liu, C. Yin, X. Liang and G. Lu, Sens. Actuators, B, 2014, 198, 26–32 CrossRef CAS PubMed.
- F. Qiu, Q. Zhu, X. Yang, Y. Quan and L. Sun, Sens. Actuators, B, 2003, 93, 237–242 CrossRef CAS.
- M. M. Rahman, S. B. Khan, M. Faisa, A. M. Asiri and K. A. Alamry, Sens. Actuators, B, 2012, 171–172, 932–937 CrossRef CAS PubMed.
- S. Vijayan, P. A. Joy, H. S. Potdar, D. Patil and P. Patil, Sens. Actuators, B, 2011, 157, 121–129 CrossRef PubMed.
- G. Gusmano, G. Montesperelli and E. Traversa, Sens. Actuators, B, 1992, 7, 460–463 CrossRef CAS.
- N. Miura, T. Raisen, G. Lu and N. Yamazoe, Sens. Actuators, B, 1998, 47, 84–91 CrossRef CAS.
- S. A. Anggraini, V. V. Plashnitsa, P. Elumalai, M. Breedon and N. Miura, Sens. Actuators, B, 2011, 160, 1273–1281 CrossRef CAS PubMed.
- G. Lu, N. Miura and N. Yamazoe, J. Mater. Chem., 1997, 7, 1445–1449 RSC.
- H. Jin, M. Breedon and N. Miura, Talanta, 2012, 88, 318–323 CrossRef CAS PubMed.
- H. Zhang, J. Li, X. Liang, G. Yin, J. Zheng and G. Lu, Sens. Actuators, B, 2013, 180, 66–70 CrossRef CAS PubMed.
- G. Lu, N. Miura and N. Yamazoe, Sens. Actuators, B, 1996, 35–36, 130–135 CrossRef.
- Y. Shimizu, H. Nishi, H. Suzuki and K. Maeda, Sens. Actuators, B, 2000, 65, 141–143 CrossRef CAS.
- V. V. Plashnitsa, V. Gupta and N. Miura, Electrochim. Acta, 2010, 55, 6941–6945 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |









