Surface modification of a PVDF membrane by cross-linked collagen
Lishun Wua,
Junfen Sun*b and
Faqin Tongb
aDepartment of Chemistry and Chemical Engineering, Heze University, Daxue Road 2269, Heze, Shandong Province 274015, P.R. China
bState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Material Science & Engineering, Donghua University, North People Rd. 2999, Songjiang, Shanghai 201620, P.R. China. E-mail: junfensun@dhu.edu.cn; Fax: +8621-67792855; Tel: +86-18602105973
First published on 19th November 2014
Abstract
A polyvinylidene fluoride (PVDF) membrane was modified by a low temperature plasma treatment and grafted with cross-linked collagen. The crosslinker of collagen was glutaraldehyde. The effects of glutaraldehyde concentration, crosslinking time and crosslinking temperature on membrane properties and surface structure were investigated. The properties of the modified membrane were characterized by the contact angle and pure water flux. The surface structure of the membrane dyed with an acid dye was observed by a polarizing microscope. The modified membrane was further characterized concerning permeability and adsorption capacity. Bovine serum albumin (BSA) was used as a model protein. The cell culture ability of the membrane was examined by the methyl thiazolyl tetrazolium (MTT) method. The amount of grafted collagen on the membrane surface reached the maximum when collagen was cross-linked with 0.4 wt% glutaraldehyde for 1 h at 35 °C. The BSA adsorption capacity and cell culture ability examination indicated that the PVDF membrane grafted with cross-linked collagen had good hydrophilicity and biocompatibility.
1. Introduction
Polyvinylidene fluoride (PVDF) is a semicrystalline polymer with repeated units of –(CH2CF2)n–. It exhibits excellent mechanical properties, corrosion and heat resistance, radiation and chemical stability. Moreover, it shows good flexibility and higher strength.1,2 Products prepared with PVDF are widely applied in many fields such as food, biological medicine and water treatment industry.3 A PVDF membrane has been widely used in membrane distillation, sewage treatment and wine filtration in the recent years. The requirements for the surface properties of a PVDF membrane are different when a PVDF membrane is applied in different fields. For example, hydrophilicity modification is used to improve antifouling property of PVDF membrane in the fields of food and water treatment in order to reduce protein adsorption of membrane surface.4 While hydrophobicity modification is needed in the field of membrane distillation.5 Moreover biocompatibility of membrane is required in the field of biological medicine.6,7 In general, the modification methods about membrane surface include blending and surface treatment.8 Plasma treatment is a common method to change the surface properties of membrane and is an easy way to treat membrane. Grafting polymerization is further induced and other functional groups are introduced on membrane surface.9–13 There are two advantages for grafting polymerization modification induced by low temperature plasma treatment. One is that the surface property does not decay for a long time. The other is that the surface property is effectively improved. PVDF membrane has been modified by Akashi12 and Yang14 by using plasma treatment to improve hydrophilicity of membrane surface. The studies show that hydroxyl, carboxyl and carbonyl groups are generated on the membrane surface. After PVDF membrane was treated by plasma, poly(acrylic acid) (PAA), immunoglobulin G (IgG), poly(glycidylmethacrylate) (PGMA) and 2-methacrylic acid 3-(bis-carboxymethylamino)-2-hydroxyl-propyl ester (GMA-IDA) were introduced on membrane surface by You,9 Paslaru,11 Young15 and Li16 respectively to improve hydrophilicity and biocompatibility of membrane. Carbon tetrafluoride (CF4) was grafted on the surface of PVDF membrane via plasma treatment by Yang17 and the modified membrane was used for direct membrane distillation.Collagen protein is a crucial biological material and mainly consists of glycocoll, proline, alanine and so on. Collagen is one of the major constituents of extracellular matrix and is well known for low antigenicity, excellent biocompatibility and biodegradability.18 It has good biocompatibility in comparison to other synthetic materials. The study on collagen protein is very active nowadays. It is widely applied in the fields of medicine and health care, cosmetics and food now. Moreover researches and applications about collagen increase gradually in the fields of nerve conduits, artificial skin, food packaging and microbiological culture media.19–22 However the application about collagen is limited due to lower mechanical strength and fast degradation in the body. So crosslinker is used to combine collagen molecules with covalent bonds to improve the stability and mechanical strength of collagen.
Glutaraldehyde is a most widely used crosslinker with two functional groups. It has good water solubility as well as lower price. Two Schiff alkalies will form by the reaction between two aldehyde groups and two amine groups in identical or different molecules, and two collagen molecules are combined by a five carbon bridges.23,24 Aldehyde group could react with residue amine, amide and other groups of collagen molecules.25 As a crosslinker, glutaraldehyde shows a lot of advantages such as high reaction activity with protein, good crosslinking performance and good stability of crosslinking points between glutaraldehyde and protein. Furthermore, glutaraldehyde combines the linear molecular chains of protein and netty molecular chains of protein form in solution, which keep the fine structure of protein molecular chains in space. The collagen cross-linked by glutaraldehyde on hydroxyapatite,26 galactomannan,27 aminolyzed poly(L-lactic acid)28 and chitosan18 was reported. However the research concerning grafting cross-linked collagen on PVDF membrane surface by plasma treatment has not been found yet. To graft cross-linked collagen on PVDF membrane surface by plasma treatment improves the hydrophilicity and biocompatibility of PVDF membrane because collagen contains hydrophilic groups. In this study, the cross-linked collagen was grafted on the surface of PVDF membrane to improve the hydrophilicity and biocompatibility of PVDF membrane. The contact angle, pure water flux, dyeing characterization, protein adsorption capacity and cell culture ability were investigated to study the influence of cross-linked collagen on the hydrophilicity and biocompatibility of modified PVDF membrane.
2. Experimental
2.1 Materials
Poly(vinylidene fluoride) (PVDF) flat membrane with reported pore size of 0.45 μm was purchased from the Millipore Co. Ltd (US). Collagen (Mn = 3000 Da) was purchased from Tianfu garden biological technology Co. Ltd (China). Helium (the purity was 99.99%) was obtained from Lmgas Co. Ltd of Shanghai (China). Acid dye (M-B) was purchased from Yayun Co. Ltd of Shanghai (China). Glutaraldehyde (analysis grade) and bovine serum albumin (BSA, Mw = 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were bought from Shanghai Chemical Reagent Company (China).
000) were bought from Shanghai Chemical Reagent Company (China).
2.2 Membrane modification method
Glutaraldehyde was added into 25 g l−1 collagen solution. The concentration range of glutaraldehyde was changed from 0 to 0.6 wt%. The crosslinking temperature was monitored from 25 °C to 55 °C and the crosslinking time was adjusted from 0 to 2 h.PVDF membrane was cut into small pieces (5 cm × 5 cm) and arranged in the chamber of low temperature plasma treatment apparatus (HD-1A, ZhongKe ChangTai Plasma Technology Co., Ltd, China). Radio frequency power and inductive coupled electrode were adopted. The treatment condition was selected as argon medium, 20 Pa gas pressure, 50 W power and 90 s processing time.
After PVDF membrane was treated by low temperature plasma treatment, it was rapidly immersed into the 25 g l−1 cross-linked collagen solution at room temperature to initiate grafting reaction. The membrane was taken out in 60 min and washed with distilled water to clean the collagen adsorbed on the membrane surface, and stored in wet state. We found 25 °C of grafting temperature and 60 min of grafting time were good for PVDF membrane in previous study. So 25 °C of grafting temperature and 60 min of grafting time was used in this work.
2.3 Pure water flux
The membranes were subjected to pure water flux estimation at a trans-membrane pressure of 0.1 MPa under cross-flow filtration. The permeability was measured under steady-state flow. Pure water flux was calculated as follows:where Q was the quantity of permeate collected (in l), and A was membrane area (m2), Δt was the sampling time (h), Jw was pure water flux (l m−2 h−1).
2.4 Contact angle
Contact angle of modified PVDF membrane was measured at room temperature (25 ± 1 °C) by using sessile drop method. A computer-controlled video contact angle meter (OCA40, Dataphysics Company, Germany) was used. Water contact angle measurements (seven measures on different positions per sample, 1 μl drops of distilled water) were carried out and each measurement was considered to have ±3° accuracy.2.5 The dyeing of membrane surface
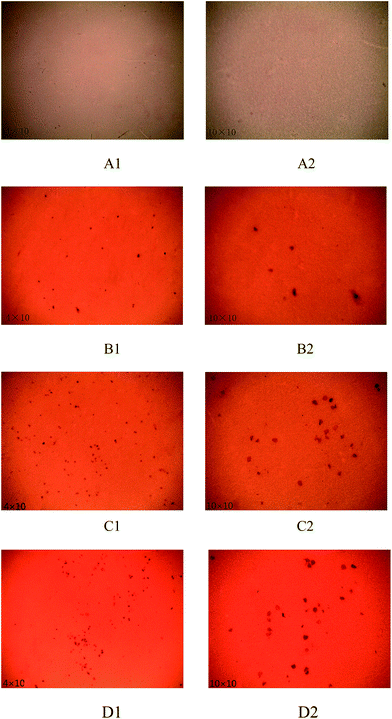
0.5 g l−1 acid dye (M-B) solution was prepared with distilled water. Grafted PVDF membrane was clipped into small pieces (1.5 cm × 2 cm) and put into the dye solution for 2 h. After the membrane was dyed completely, it was taken out and washed with distilled water. The membrane was soaked up with filter paper and photographs of the surface of dyed membranes were taken on a Leica DM750P polarizing microscope (Germany).2.6 BSA static adsorption
The static protein adsorption capacity of membranes was determined with bovine serum albumin (BSA). The membranes were dried at 30 °C in a vacuum oven before examination. The samples containing 2 g l−1 BSA were incubated with an exact amount of membranes in sealed containers under continuous shaking at 25 °C. The membrane adsorbed the BSA thereby reducing the BSA concentration in the bulk. The equilibrium BSA concentration after 24 h was monitored in time with a UV-1800 spectrophotometer which was produced by SHIMADZU Company. The BSA depletion was measured at 280 nm with 5 mm quartz cuvettes.2.7 Cell culture ability examination
Cell culture ability of PVDF membrane was evaluated by methyl thiazolyl tetrazolium (MTT) method. The experimental process was described as follow: (1) the PIEC cells (pig artery endothelial cells) in the wall of the culture vessel were digested by trypsin. (2) The cells were counted with the method of blood cell counting plate and were suspended in water to achieve appropriate concentration. (3) A piece of membrane and PIEC cells were put into the holes of a 96 holes plate. The number of PIEC cells was ten thousands per hole. Fresh nutrient solution was added in the holes until the total bulk of each hole was 200 μl. (4) The 96 holes plate was put in incubator for overnight at 37 °C. (5) The nutrient solution was changed and the total volume of each hole was kept as 200 μl. (6) The 96 holes plate was cultured in incubator for 24 h at 37 °C. (7) The liquids in the 96 holes plate were poured out. 1 μl MTT was added in each hole and the plate was cultured in incubator for 4 h at 37 °C. (8) 100 μl DMSO was added and was shaken for 20 min. (9) The optical density value was test by an enzyme standard instrument (MUTISKAN, Labsystems company, Finland) at 570 nm. The enzyme standard instrument was used to detect samples (less than 250 μl) on a 96 holes plate with colorimetric method.3. Results and discussions
3.1 Effect of glutaraldehyde concentration on properties and surface structure of modified PVDF membrane
After PVDF membrane is modified by low temperature plasma treatment, there are a large number of free radicals such as hydroxyl, carbonyl, carboxyl and other polar groups on the membrane surface.12 Collagen protein molecule possesses terminal amine and carboxyl groups. Generally collagen protein adopts two ways to be grafted on the membrane surface. The first way is that part of the radical groups on membrane surface could transfer to protein to form protein free radicals.29,30 The protein free radicals react with radical groups on membrane surface and protein is grafted on membrane surface because of coupling termination. The second way is that the unreacted amine groups on collagen molecules react with the carbonyl groups on membrane surface and collagen molecule is introduced on the surface of membrane.12
The intramolecular and intermolecular crosslinking points form between glutaraldehyde molecule and collagen molecule after collagen is cross-linked by glutaraldehyde. Two aldehyde groups at both ends of the glutaraldehyde form Schiff bases with amine groups of collagen molecules respectively. The two Schiff bases with five carbon bridges connect collagen molecules so that the molecular weight of collagen becomes bigger and the stability of collagen is strengthened. The chemical equation of the reaction is as follows:
The quantity of collagen grafted on each active centre of membrane surface increases when membrane surface is grafted with cross-linked collagen. The contact angle of membrane decreases and water flux increases because of the good hydrophilicity of collagen. When glutaraldehyde concentration is 0.4 wt%, collagen protein grafted on membrane surface reaches a maximum value, which leads to a minimum contact angle and a maximum water flux at this point. However the contact angle increases and water flux decreases when the glutaraldehyde concentration further increases. This is attributed to two reasons. Firstly, when glutaraldehyde concentration is over 0.4 wt%, the number of terminal amine groups of collagen protein molecules cross-linked with glutaraldehyde increases with the increasing glutaraldehyde concentration, which results in excessive crosslinking reaction and high crosslinking degree. Moreover the residual quantity of terminal amine groups decreases and the number of reactive centre reacting with the carboxyl groups on membrane surface reduces so that the quantity of collagen introduced onto membrane surface decreases. Secondly, collagen protein molecules may turn into dense cluster structures because of excessive crosslinking reaction when glutaraldehyde concentration is over 0.4 wt%. The remaining reactive terminal amine groups are wrapped up in the cluster and the number of reactive centre of the cross-linked collagen decreases, which makes less amount of collagen be grafted on PVDF membrane surface. So with further increasing glutaraldehyde concentration, the quantity of collagen grafted on membrane surface decreases, the hydrophilicity of PVDF membrane decreases and the contact angle increases.
A large number of active centres such as free radicals and carbonyl groups form on membrane surface after PVDF membrane is modified by low temperature plasma treatment. It is possible that each active centre has a chance to react with one collagen molecule during collagen grafting reaction process. The uncross-linked collagens have low molecular weight. The collagens with low molecular weight are introduced on the active centre of membrane surface when the collagens are not cross-linked by glutaraldehyde. Collagen molecular weight increases several times after crosslinking. The collagens with high molecular weight are introduced on active centres of membrane surface during grafting reaction process when the collagens are cross-linked. However it is difficult to graft excessively cross-linked collagens on membrane surface. The above phenomenon is observed directly after membrane surfaces are dyed.
3.2 Effect of crosslinking time on properties and surface structure of modified PVDF membrane
The aldehyde groups of the glutaraldehyde react with the terminal amine groups of collagen and Schiff bases form during crosslinking reaction process of collagen and glutaraldehyde. Consequently, covalent bonds form among collagen molecular chains, which results in high molecular weight and stability of collagen. However the reaction rate is slow because of low concentrations of collagen and glutaraldehyde in solution.
The crosslinking degree of collagen increases and the molecular weight of cross-linked collagen increases gradually with extending crosslinking time. The amount of collagen introduced on membrane surface during grafting reaction process gradually increases so that the contact angle of membrane decreases and pure water flux increases when crosslinking time is less than 1 h. While the crosslinking degree of collagen is too high and the dense clusters form when crosslinking time is over 1 h. The number of remaining terminal amine groups and the functional groups participated in grafting reaction decrease, and most of the remaining terminal amine groups of collagen are wrapped up in the clusters. The amine groups embedded in the clusters are difficult to react with the active centres on membrane surface, which results in less amount of collagen grafted on membrane surface. Therefore, the contact angle of membrane increases and pure water flux decreases.
3.3 Effect of crosslinking temperature on properties and surface structure of modified PVDF membrane
When collagen is cross-linked with glutaraldehyde at 35 °C which is close to the temperature of human body (37 °C), the molecular chains of collagen elongate and do not shrink in solution. The crosslinking density of molecular chains of collagen was improved and the elongation state of molecular chains of collagen was solidified in solution by using glutaraldehyde to react with collagen. Moreover further grafting reaction of residual active centre is not affected and good graft ratio is kept. Therefore the contact angle of modified PVDF membrane reaches the minimum value and pure water flux reaches the maximum value when crosslinking temperature is 35 °C. When the crosslinking temperature rises to 45 °C and 55 °C, the rate of crosslinking reaction is accelerated. More terminal amine groups participate in crosslinking reaction during reaction process, which leads to excessive crosslinking reaction and a dense cluster structure of collagen. That the residual terminal amine groups are wrapped up in the cluster and block further grafting reaction successfully reduces the ratio of grafting reaction and the amount of collagen on membrane surface. Meanwhile, it is even possible that the denaturation of collagen protein happens at higher temperature and the hydrophilicity of collagen protein becomes worse. Accordingly, the contact angle of PVDF membrane increases and pure water flux decreases rapidly with further rising crosslinking temperature.
3.4 BSA static adsorption
Table 1 shows the BSA adsorption capacity of different membranes at pH 6. PVDF is the original PVDF membrane, G-PVDF is the membrane grafted with uncross-linked collagen after plasma treatment, and C-PVDF is the membrane grafted with cross-linked collagen after plasma treatment. The difference between G-PVDF and C-PVDF is that the collagens on the surface of PVDF membrane are cross-linked with glutaraldehyde for different time. The crosslinking time of G-PVDF and C-PVDF is 0 and 1 h respectively. As shown in Table 1, for BSA adsorption capacity, C-PVDF < G-PVDF < PVDF. It means that for hydrophilicity, C-PVDF > G-PVDF > PVDF. The low BSA adsorption capacity contributes to high biocompatibility of C-PVDF membrane.| Membrane | PVDF | G-PVDF | C-PVDF |
|---|---|---|---|
| Adsorption capacity (mg g−1 membrane) | 3.43 | 2.83 | 1.81 |
3.5 Cell culture ability examination
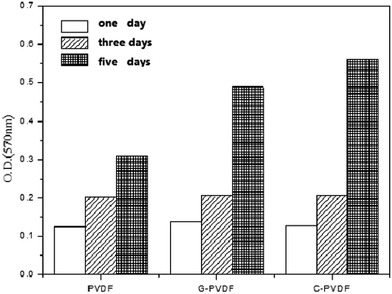
MTT method is adopted to examine the ability of membranes on cell culturing. The OD values of different PVDF membranes are shown in Fig. 7. It can be seen from Fig. 7 that the quantity of cells on three different membranes increases with extending culturing time. The growth rate of cells is different for different PVDF membranes. In the first 3 days, the growth rate and quantity of cells on three kinds of membrane are almost the same. In five days, the optical density value (OD value) of different membranes is much different. The OD values of original PVDF membrane, PVDF membrane grafted with uncross-linked collagen (G-PVDF) and PVDF membrane grafted with cross-linked collagen (C-PVDF) are 0.309, 0.489 and 0.562 respectively. It indicates that the growth rate and quantity of cells cultured on C-PVDF membrane have the maximum value among three membranes. High OD value means good cells activity and strong cells culture ability of PVDF membrane. It is suggested that C-PVDF possesses the strongest ability for cell culturing and the best biocompatibility among three different PVDF membranes.4. Conclusions
PVDF membrane modified by low temperature plasma treatment is used to graft collagen cross-linked by glutaraldehyde. Contact angle reaches the minimum value of 55.6° and pure water flux reaches the maximum value of 271.9 l m−2 h−1 when collagen is cross-linked with 0.4 wt% glutaraldehyde concentration for 1 h at 35 °C. At this point, the amount of collagen grafted on membrane surface is the highest. For BSA static adsorption capacity, C-PVDF < G-PVDF < PVDF. For hydrophilicity and biocompatibility of membranes, C-PVDF > G-PVDF > PVDF. C-PVDF membrane possesses the strongest ability for cell culturing and the best biocompatibility compared to original PVDF membrane and G-PVDF membrane.Acknowledgements
The authors thank National Natural Science Foundation of China (51203020); Scientific Research Starting Foundation for Returned Overseas Chinese Scholars, Ministry of Education of China; Donghua University central university scientific research special fund (2232012D3-32); Shandong Nature Science Foundation (BS2013HZ027).References
- S. Rajabzadeh, C. Liang, Y. Ohmukai, T. Maruyama and H. Matsuyama, J. Membr. Sci., 2012, 423–424, 189 CrossRef CAS PubMed.
- Z. L. Cui, N. T. Hassankiadeh, S. Y. Lee, K. T. Woo, J. M. Lee, A. Sanguineti, V. Arcella, Y. M. Lee and E. Drioli, J. Membr. Sci., 2015, 473, 128 CrossRef CAS PubMed.
- C. Y. Feng, K. C. Khulbe, T. Matsuura and A. F. Ismail, Sep. Purif. Technol., 2013, 111, 43 CrossRef CAS PubMed.
- C. Q. Zhao, X. C. Xu, J. Chen and F. L. Yang, Int. J. Chem. Environ. Eng., 2013, 1, 349 CrossRef CAS PubMed.
- Z. Y. Wang, L. Q. Sun, Q. Wang, B. A. Li and S. C. Wang, Eur. Polym. J., 2014, 60, 262 CrossRef CAS PubMed.
- J. Yuan, J. Q. Meng, Y. L. Kang, Q. Y. Du and Y. F. Zhang, Appl. Surf. Sci., 2012, 258, 2856 CrossRef CAS PubMed.
- Y. Chang, Y. J. Shih, R. Ruaan, A. Higuchi, W. Chen and J. Lai, J. Membr. Sci., 2008, 309, 165 CrossRef CAS PubMed.
- F. Liu, N. A. Hashim, Y. T. Liu, M. R. Moghareh Abed and K. Li, J. Membr. Sci., 2011, 375, 1 CrossRef CAS PubMed.
- S. You, G. U. Semblante, S. Lu, R. A. Damodar and T. Wei, J. Hazard. Mater., 2012, 237–238, 10 CrossRef CAS PubMed.
- C. Yao, X. S. Li, K. G. Neoh, Z. L. Shi and E. T. Kang, Appl. Surf. Sci., 2009, 255, 3854 CrossRef CAS PubMed.
- E. Paslaru, M. C. Baican, E. G. Hitruc, M. T. Nistor, F. Poncin-Epaillard and C. Vasile, Colloids Surf., B, 2014, 115, 139 CrossRef CAS PubMed.
- N. Akashi and S. Kuroda, Polymer, 2014, 55, 2780 CrossRef CAS PubMed.
- Y. Chang, C. Y. Ko, Y. Shih, D. Quemener, A. Deratani, T. Wei, D. Wang and J. Lai, J. Membr. Sci., 2009, 345, 160 CrossRef CAS PubMed.
- X. Yang, R. Wang, L. Shi, A. G. Fane and M. Debowski, J. Membr. Sci., 2011, 369, 437 CrossRef CAS PubMed.
- T. Young, H. Chang, D. Lin and L. Cheng, J. Membr. Sci., 2010, 350, 32 CrossRef CAS PubMed.
- S. Li, C. Wang and C. Chen, J. Membr. Sci., 2008, 318, 429 CrossRef CAS PubMed.
- C. Yang, X. Li, J. Gilron, D. Kong, Y. Yin, Y. Oren, C. Linder and T. He, J. Membr. Sci., 2014, 456, 155 CrossRef CAS PubMed.
- Y. Liu, L. Ma and C. Gao, Mater. Sci. Eng., C, 2012, 32, 2361 CrossRef CAS PubMed.
- A. Lionel, A. Michael and R. Tomas, Biomaterials, 2011, 32, 1543 CrossRef PubMed.
- T. Takahashi, T. Kondo, K. Tanaka, S. Hattori, S. Irie, S. Kudoh, S. Imura and H. Kanda, Polym. Degrad. Stab., 2012, 97, 1002 CrossRef CAS PubMed.
- C. Huang, Y. Chien, T. Ling, H. Cho, J. Yu and Y. Chan, Biomaterials, 2010, 31, 8271 CrossRef CAS PubMed.
- R. R. Rao, A. Jiao, D. H. Kohn and J. P. Stegemann, Acta Biomater., 2012, 8, 1560 CrossRef CAS PubMed.
- A. K. Dutta, A. Nayak and G. Belfort, J. Colloid Interface Sci., 2008, 324, 55 CrossRef CAS PubMed.
- S. B. Adeloju and A. T. Lawal, Anal. Chim. Acta, 2011, 691, 89 CrossRef CAS PubMed.
- S. Farris, K. M. Schaich, L. Liu, P. H. Cooke, L. Piergiovanni and K. L. Yam, Food Hydrocolloids, 2011, 25, 61 CrossRef CAS PubMed.
- M. Kikuchi, H. N. Matsumoto, T. Yamada, Y. Koyama, K. Takakuda and J. Tanaka, Biomaterials, 2004, 25, 63 CrossRef CAS.
- S. D. Figueiro, J. C. Goes, R. A. Moreira and A. S. B. Sombra, Carbohydr. Polym., 2004, 56, 313 CrossRef CAS PubMed.
- Y. Zhu and M. B. Chan-Park, Anal. Biochem., 2007, 363, 119 CrossRef CAS PubMed.
- A. S. Domazou, V. Zelenay, W. H. Koppenol and J. M. Gebicki, Free Radical Biol. Med., 2012, 53, 1565 CrossRef CAS PubMed.
- Z. Yu, Xi. Li, X. Wang, X. Ma, X. Li and K. Cao, J. Chem. Sci., 2012, 124, 537 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |