End-group differentiating ozonolysis of furocoumarins†
Mikhail V. Malakhova,
Maxim A. Dubinnyib,
Natalia V. Vlasovaa,
Victor G. Zgodac,
Roman G. Efremovb and
Ivan A. Boldyrev*ab
aPirogov Russian National Research Medical University, ul. Ostrovityanova 1, Moscow 117997, Russia. E-mail: malakhov.mikhail@gmail.com; Tel: +7-916-815-5258
bShemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, ul. Miklukho-Maklaya 16/10, Moscow 117997, Russia. E-mail: ivan@lipids.ibch.ru; Tel: +7-926-224-68-06
cOrekhovich Institute of Biomedical Chemistry of the Russian Academy of Medical Sciences, ul. Pogodinskaya 10, Moscow 119121, Russia. E-mail: vic_zgoda@yahoo.com; Tel: +7-916-359-2656
First published on 10th November 2014
Abstract
Ozonolysis of furocoumarins followed by reductive work-up yields not only common symmetrical dialdehydes, but also o-formylumbelliferones with moderate-to-high yields. Simultaneous formation of both products accounts for the transformation of carbonyl oxides – products of primary ozonide ring opening.
Ozonolysis is widely applied in organic synthesis (reviews1). Briefly, upon treatment with ozone a double bond oxidatively degrades through the stage of primary ozonide (1,2,3-trioxolane) yielding an aldehyde and a very reactive carbonyl oxide (Criegee intermediate, reviews2). In a non-participating solvent, it recomposes with aldehyde to form secondary ozonide (1,2,4-trioxolane), which upon reduction yields two aldehydes or, in the case of cyclic or polycyclic structures, two aldehyde groups within a molecule. This is usually unwanted since it is difficult to treat selectively only one of two aldehyde groups during further compound transformations. Ozonolysis of the terminal double bond with a formation of volatile formaldehyde as a by-product is used to introduce a single aldehyde group (see ref. 3 for the recent examples of this approach). In turn, short-lived carbonyl oxides can be scavenged by a participating solvent (e.g. methanol) resulting in alkoxyhydroxyperoxides, which can be further converted to esters. Thus, double bond ozonolysis may serve as a beneficial approach to introduce into a molecule two different functional groups that can be further independently modified. In complicated syntheses, such simultaneous introduction of two groups may dramatically reduce the number of steps (see ref. 4 for the recent examples). In contrast to reactions yielding symmetrical dialdehydes, such approach was referred to as “end-group differentiating ozonolysis” as proposed originally by Carreira et al.4e (also known as “unsymmetrical” or “non-symmetric” ozonolysis in earlier works4g–i). Here, we performed the end-group differentiating ozonolysis of furocoumarins, which are known natural photosensitizers and widely used in biomedical chemistry.
o-Formylumbelliferones (o-formyl-7-hydroxycoumarins) were found to be apoptogenic for T lymphoblastic Jurkat cells5 and are used as fluorescence turn-on probes for homocysteine and cysteine in aqueous solutions at neutral pH.6 Earlier, it was reported that ozonolysis of 8-methoxypsoralen led to 6-formyl-8-methoxyumbelliferone with moderate yields (see ref. 7 for the examples), and we extended this approach for some other furocoumarins.
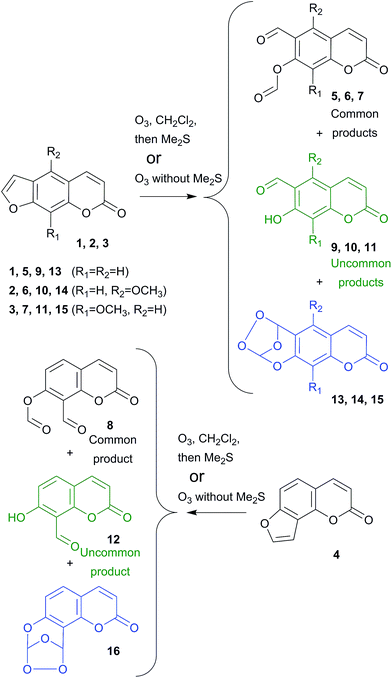
Upon ozonolysis of different furocoumarins (Scheme 1, 1–4) in CH2Cl2 at −84 °C with subsequent dimethyl sulfide (Me2S) reductive work-up,‡ we obtained a mixture of two products.§ The first product is a symmetrical dialdehyde compound, and the only product expected upon ozonolysis in a non-participating solvent (common product). The second one (an uncommon product) is the respective o-formylumbelliferone initially accounted for as the product of dialdehyde hydrolysis after reductive work-up. We allowed the reaction mixture to stay in the dark at ambient temperature monitoring the conversion of dialdehydes 5–8 to o-formylumbelliferones 9–12.¶ Surprisingly, such a conversion required days or even weeks: in a mixture of 5 and 9 it took one month to convert most of 5 to 9 (see ESI Fig. S1† for NMR kinetics), and the conversion of 6 to 10 took about 8 days. Thus, it is unlikely that o-formylumbelliferones are formed due to hydrolysis of corresponding dialdehydes. Moreover, the slow conversion indicates that o-formylumbelliferones found in the reaction mixture an hour after the end of ozonolysis process are formed simultaneously with dialdehydes. Furthermore, it was noted that the percentage of common and uncommon products depended on the structure of the furocoumarin. Psoralen 1 lacking any substituents mainly provided the common dialdehyde (67%), with only 37% of o-formylumbelliferone. Angular structure (as for angelicin 4) or methoxy substituents (as for 2 and 3) promoted unusual product formation, and ozonolysis of 8-methoxypsoralen 3 yielded mainly the uncommon product. Thus, we state the evidence of end-group differentiating ozonolysis in the absence of carbonyl oxide scavengers.
More surprisingly, both umbelliferones and corresponding formats were obtained by ozonolysis of furocoumarins even without the following reductive work-up step, and the reaction mixture further contained the corresponding secondary ozonide.|| After analysis of the product compositions presented in Table 1 we concluded that both products could be formed directly from carbonyl oxide intermediates. These considerations are rationalized on Scheme 2 only changing parts of molecules are shown; the unfolded schemes for all furocoumarins 1–4 are provided in ESI Schemes S1–S4.†
| Source | Products | |||
|---|---|---|---|---|
| 1,2,4-Trioxolane | Formate (common) | Umbelliferone (uncommon) | Formic acid (by-product) | |
| a Also, see annotated spectra on ESI Fig. S2–S5. | ||||
| Ozone followed by Me2S workup | ||||
| 1 (Psoralen) | 0 | 63 | 37 | Not found |
| 2 (5-MOP) | 0 | 31 | 69 | Present |
| 3 (8-MOP) | 0 | 3 | 97 | Not found |
| 4 (Angelicin) | 0 | 12 | 88 | Not found |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Ozone without subsequent Me2S workup | ||||
| 1 (Psoralen) | 45 | 5 | 50 | Not found |
| 2 (5-MOP) | 6 | 63 | 31 | Present |
| 3 (8-MOP) | 29 | 37 | 34 | Present |
| 4 (Angelicin) | 36 | 4 | 60 | Present |
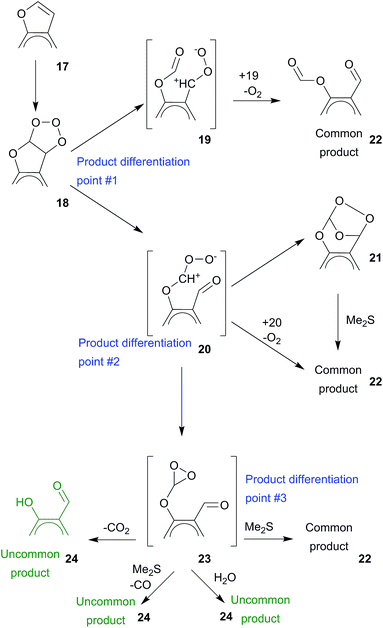
Conventionally, the initial product of ozone 3 + 2 cycloaddition to a furan 17 double bond is primary ozonide 18, which is unstable and readily decomposes to an aldehyde and carbonyl oxide. Furocoumarin structure dictates the possibility of two-way opening of primary ozonide which may result in the formation of carbonyl oxides 19 and 20. Commonly, they recombine with an aldehyde group to secondary ozonide 21. In the case of carbonyl oxides 19 and 20 the formation of 21 is slowed by the steric hindrance caused by rigidity of the aromatic ring. Moreover, carbonyl oxide 20 unlikely forms 21, since in that case it should react with an ester.2c The secondary ozonides of furocoumarins were stable for several hours on air at ambient temperature, and we obtained their 1H NMR spectra (or full sets of 2D NMR spectra for psoralen 1 and 8-methoxypsoralen 3 secondary ozonides). In the presence of reduction agent the secondary ozonides convert to corresponding common product 22, thus no traces of the secondary ozonide could be found in reaction mixtures after reductive work-up (see Table 1 and ESI Fig. S2–S5†).
The slowed formation of 21 due to steric hindrance makes the competitive pathways of carbonyl oxide decomposition possible. Two molecules of carbonyl oxide 19 can interact to form 22 with the release of one O2 molecule. This type of carbonyl oxide reaction is well-known,2c while detailed investigations of this reaction are rare due to its high speed (see ref. 8 for the recent study). Furthermore, since the formation of 21 from 19 is unlikely, the bimolecular reaction resulting information of 22 and O2 release becomes predominant for 19. The carbonyl oxide 20 can also participate in similar reaction. At the same time, the carbonyl oxides can convert to dioxiranes, either photochemically9a or thermally as known for the carbonyl oxides with α-methoxy9b or α-amino9c substituents and for diaryl carbonyl oxides.9d Carbonyl oxide 20 has phenyloxy substituent and likely converts to dioxirane 23. Thus, the formation of dioxirane 23 competes with formation of secondary ozonide 21. Dioxirane 23 being very labile may readily decompose to uncommon product 24, both in absence (with CO2 release) in presence (with CO release) of Me2S (see ref. 2d and ref. 14, 16, 20–23, and 30–33 cited therein), and we suggest that 23 is most likely to be the product providing the uncommon product 24 even without reduction work-up.
The direction of primary ozonide opening depends mostly on inductive effect of substituents at trioxolane ring. Briefly, in the presence of electron donating group primary ozonide opens with the formation of carbonyl oxide adjacent to that group. On the contrary, the opening in the presence of electron withdrawing group results in the formation of carbonyl oxide located away from that group. This scheme works well in a lot of cases (see ref. 4c for a recent example), but is not easily applicable in the case of title compounds. Both carbon atoms in trioxolane ring of 18 have electron withdrawing substituents – the furan oxygen atom and benzene ring. In addition, the said substituents depend from each other, and both depend on lactone ring and presence/position of methoxy groups. Notably, in the case of 3, methoxy group can stabilize carbonyl oxide (see ESI Scheme S3†). This is very similar to stabilizing effect of benzyloxy group on carbonyl oxide in sterically hindered intermediates (see Scheme 5 (ref. 4e)), and explains why ozonolysis of 3 followed by reduction work-up yields almost exclusively uncommon product 11 (see Table 1 and ESI Fig. S4†), whereas for other furocoumarines such predominance was not noticed.
Thus, there are three product differentiation points governing the product distribution (Scheme 2). The first one is a direction of primary ozonide 18 opening. The second is a way by which carbonyl oxide 20 may further transform either to secondary ozonide 21 or to dioxirane 23. Finally, dioxirane 23 can be reduced to common product 22 or decompose to uncommon product 24, the latter process being dependent on coumarin structure (see ESI† unfolded schemes 1–4).
Here, using furocoumarins as a substrate we report a new end-group differentiating ozonolysis reaction lacking common process of carbonyl oxide scavenging and providing o-formylumbelliferones with moderate-to-high yields. The unusual stability of furocoumarins' secondary ozonides made it possible to characterize the product compositions and propose the mechanism of furocoumarin ozonolysis despite the presence of three possible product differentiation points.
Acknowledgements
The work was supported by RFBR grant no. 13-04-40326-H. Authors would like to thank Dr A. Fedorov for helpful discussion, Dr G. Ishmuratov for valuable comment on carbonyl oxide life-time, and Natalia Kuznetsova for assistance in the manuscript preparation. Access to computational resources of the Joint supercomputer centre RAS is greatly appreciated.Notes and references
- (a) S. G. Van Ornum, R. M. Champeau and R. Pariza, Chem. Rev., 2006, 106, 2990 CrossRef CAS PubMed; (b) G. Y. Ishmuratov, Y. V. Legostaeva, L. P. Botsman and G. A. Tolstikov, Russ. J. Org. Chem., 2010, 46, 1593 CrossRef CAS.
- (a) R. Criegee, Angew. Chem., Int. Ed., 1975, 14, 745 CrossRef; (b) W. Sander, Angew. Chem., Int. Ed., 1990, 29, 344 CrossRef; (c) W. Bunnelle, Chem. Rev., 1991, 91, 335 CrossRef CAS; (d) J. M. Anglada, J. Gonzalez and M. Torrent-Sucarrat, Phys. Chem. Chem. Phys., 2011, 13, 13034 RSC; (e) L. Vereecken, H. Harder and A. Novelli, Phys. Chem. Chem. Phys., 2014, 16, 4039 RSC; (f) M. Kumar, D. H. Busch, B. Subramaniam and W. H. Thompson, J. Phys. Chem. A, 2014, 118, 1887 CrossRef CAS PubMed.
- (a) P. Cheng, D. Clive, S. Fernandopulle and Z. Chen, Chem. Commun., 2013, 49, 558 RSC; (b) A. Arlt, S. Benson, S. Schulthoff, B. Gabor and A. Furstner, Chem.–Eur. J., 2013, 19, 3596 CrossRef CAS PubMed.
- (a) S. P. Chavan, P. B. Lasonkar and P. N. Chavan, Tetrahedron: Asymmetry, 2013, 24, 1473 CrossRef CAS PubMed; (b) S. L. Schreiber, R. E. Claus and J. Reagan, Tetrahedron Lett., 1982, 23, 3867 CrossRef CAS; (c) S. A. Testero, M. I. Mangione, A. G. Suarez and R. A. Spanevello, Eur. J. Org. Chem., 2013, 5236 CrossRef CAS; (d) G. M. Chinigo, A. Breder and E. M. Carreira, Org. Lett., 2011, 13, 78 CrossRef CAS PubMed; (e) A. Breder, G. M. Chinigo, A. W. Waltman and E. M. Carreira, Angew. Chem., Int. Ed., 2008, 47, 8514 CrossRef CAS PubMed; (f) A. Breder, G. M. Chinigo, A. W. Waltman and E. M. Carreira, Chem.–Eur. J., 2011, 17, 12405 CrossRef CAS PubMed; (g) S. A. Testero and R. A. Spanevello, Org. Lett., 2006, 8, 3793 CrossRef CAS PubMed; (h) S. A. Testero, M. I. Mangione, A. A. Poeylaut-Palena, M. González Sierra and R. A. Spanevello, Tetrahedron, 2007, 63, 11410 CrossRef CAS PubMed; (i) S. A. Testero, R. A. Spanevello and R. Kohli, ARKIVOC, 2003, X, 220 CrossRef.
- S. Caffieri, F. Di Lisa, F. Bolesani, M. Facco, G. Semenzato, F. Dall'Acqua and M. Canton, Blood, 2007, 109, 4988 CrossRef CAS PubMed.
- K. S. Lee, T. K. Kim, J. H. Lee, H. J. Kim and J. I. Hong, Chem. Commun., 2008, 6173 RSC.
- (a) N. Murakami, et al., Chem. Pharm. Bull., 1991, 39, 2715 CrossRef CAS; (b) M. E. Brokke and B. E. Christensen, J. Org. Chem., 1958, 23, 589 CrossRef CAS.
- Y.-T. Su, H.-Y. Lin, R. Putikam, H. Matsui, M. C. Lin and Y.-P. Lee, Nat. Chem., 2014, 6, 477 CrossRef CAS PubMed.
- (a) W. Sander, K. Schroeder, S. Muthusamy, A. Kirschfeld, W. Kappert, R. Boese, E. Kraka, C. Sosa and D. Cremer, J. Am. Chem. Soc., 1997, 119, 7265 CrossRef; (b) K. R. Kopecky, Y. Xie and J. Molina, Can. J. Chem., 1993, 71, 272 CrossRef CAS; (c) K. Ishiguro, K. Hirabayashi, T. Nojima and Y. Sawaki, Chem. Lett., 2002, 796 CrossRef CAS; (d) H. Hanaki, Y. Fukatsu, M. Harada and Y. Sawak, Tetrahedron Lett., 2004, 45, 5791 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Annotated NMR data and raw 1D/2D spectra, geometry and charge distribution in 1,2,4-trioxolane intermediates. See DOI: 10.1039/c4ra08106d |
| ‡ General synthetic procedure: solution of furocoumarin (10–30 mg) in dry CH2Cl2 was bubbled with ozone-enriched oxygen at −84 °C (ethyl acetate/liquid nitrogen bath) until becoming slightly blue – usually in about 20 min. (At this point reaction mixture could contain yellow precipitate). After removing excess ozone with a stream of nitrogen (5 min), 50 μl of Me2S was added, and the reaction mixture was allowed to warm to ambient temperature. The solution was evaporated and re-dissolved in CDCl3 for analysis. |
| § Compounds lacking R1 substituent (Scheme 1) appeared to be very labile. The mixture of 6 and 10 yields insoluble solid upon storage at 4 °C in a week. Being exposed to light, the mixture of 5 and 9 also yields similar insoluble solid, and thus should be processed in dark. While compounds 7 and 8 could be purified by chromatography, compounds 5 and 6 decompose during this procedure. Compounds bearing R1 substituent are stable. |
| ¶ The sample for NMR kinetics studies was prepared mostly as described in ‡. After warming up, it was mixed with equal volume of water and left stirring in the dark. At times, aliquots were collected, evaporated, and re-dissolved in CDCl3 for analysis. |
| || Ozonolysis without Me2S work-up was done with following changes as compared to ‡. The reaction was run in CDCl3 at −44 °C (acetonitrile/liquid nitrogen bath). After bubbling with nitrogen, the reaction mixture was allowed to warm up to ambient temperature. The white precipitate was centrifuged off, and the supernatant was transferred to NMR analysis. The ozonolysis of 3 yields negligible amount of insoluble material, whereas the ozonolysis of 1, 2 and 4 yields sufficient amount of the insoluble material, which considered to be a result of product polymerization. Samples containing trioxolanes were moderately stable, and we were able to get the full set of 2D NMR spectra before product decomposition. |
| This journal is © The Royal Society of Chemistry 2014 |