Synthesis of imidazolidin-2-ones employing dialkyl carbonates as an ecofriendly carbonylation source†
Abstract
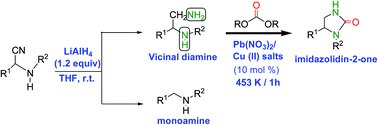
A new approach to the synthesis of imidazolidin-2-ones by carbonylation of vicinal diamines with dialkyl carbonates using Pb(NO3)2 and Cu(II) salts as catalysts has been described in the present protocol. A comparative study using Cu(II) salts and Pb(NO3)2 as catalysts has suggested Cu(NO3)2 and CuCl2·2H2O salts to be as promising as Pb(NO3)2 and can replace the latter in the carbonylation reactions employing dialkyl carbonates.

 Please wait while we load your content...
Please wait while we load your content...