Organic amine-functionalized silica-based mesoporous materials: an update of syntheses and catalytic applications
Abstract
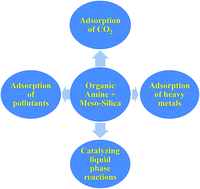
Nowadays, inorganic–organic hybrid materials having pores in the mesoporous range are an intensively studied new category of demanding materials. By template synthesis, the coupling of inorganic and organic components gives pore sizes between 2 and 15 nm with very high surface area. The inorganic–organic hybrid materials were prepared in two ways: one was by co-condensation and the other by post-synthesis method. The inorganic part provides mechanical strength and the organic part shows functional activities. This review gives an overview of the preparation, properties, and potential applications of these materials in the areas of adsorption of pollutant gases like CO2 and heavy metals and in catalysis. Their activity is found to be very impressive in all these fields and it is hoped to be improved in the near future.

 Please wait while we load your content...
Please wait while we load your content...