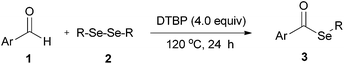
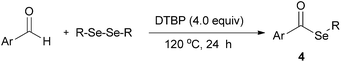
Syntheses of selenoesters through C–H selenation of aldehydes with diselenides under metal-free and solvent-free conditions†
Jyun-Cyuan Liou‡
,
Satpal Singh Badsara‡,
Yu-Ting Huang and
Chin-Fa Lee*
Department of Chemistry, National Chung Hsing University, Taichung, Taiwan 402, Republic of China. E-mail: cfalee@dragon.nchu.edu.tw; Fax: +886-4-2286-2547
First published on 26th August 2014
Abstract
A DTBP-promoted C–H selenation of aldehydes with diselenides under metal-free and solvent-free conditions is described. This system shows good functional group compatibility, functional groups including bromo, trifluoromethyl, chloro, amine and heterocycle-containing moieties including thiophene and furan are all tolerated by the reaction conditions employed. Both diaryl and dialkyl diselenides reacted smoothly with aldehydes to provide selenoesters in good to excellent yields.
Introduction
Recently, organoselenium compounds have gained much attention due to their applications in the fields of chemical biology,1 asymmetric catalysis,2,3 cross-coupling reactions,4 organic synthesis,5–7 materials science,8 and natural products.9 Many methods have been reported for preparing selenoesters,10–13 and the transition-metal-catalyzed cross-coupling reaction of acyl chlorides with nucleophilic selenium reagents is one of the most popular approaches for preparing such molecules.12,13 Transition metals including Hg,12a Pd,12d,13a Fe,12g Sm,13b In,12f,13c,13d Rh,13e Cu,13f Zn,13g La13h have been used as the catalysts for this transformation. However, the above methods suffered from some synthetic drawbacks due to the issue of air stability and/or toxicity of the transition metals, starting materials and solvents; moreover, these systems usually used not easily available selenium containing precursors.10a,12a,12d,14a Recently, organo-catalysis15 has been employed as an alternative choice to replace transition metal mediated transformations,10 and application of this approach to the direct C–H bond selenation of aldehydes with diselenides has received less attention.10c Very recently, we have reported the DTBP-promoted cross-coupling of aldehydes with disulfides to provide thioesters under metal-free conditions.15a Here, we report that the selenoesters can be prepared through the coupling of aldehydes with diselenides in the presence of DTBP as an oxidant without transition metals under solvent-free conditions.Results and discussion
Initially, 4-methoxybenzaldehyde (1a) and diphenyl diselenide (2a) were chosen as the coupling partners to determine the optimal reaction conditions. The results are summarized in Table 1. Only trace amount of the selenoester 3a was obtained when the reaction was carried out by using TBHP (tert-butyl hydroperoxide) as an oxidant14b (Table 1, entry 1). Interestingly, a 64% yield of the target was obtained when the reaction was carried using TBPB (tert-butyl peroxybenzoate) as oxidant (Table 1, entry 2). Based on this result, we screened other oxidants (Table 1, entries 3–8), and DTBP was found to be the best, giving 3a in 93% yield (Table 1, entry 8).16 It was also found that lower amounts of DTBP (Table 1, entries 9 and 10), lower reaction temperatures (Table 1, entries 11 and 12) and shorter reaction time (Table 1, entry 13) diminished the yield of the product. Lower amount of 4-methoxybenzaldehyde will reduce the yield of the product (59%) (Table 1, entry 14).| Entry | Oxidant (equiv.) | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 4-methoxybenzaldehyde (1.0 mL), diphenyl diselenide (0.25 mmol) and oxidant (2.0 mmol) were reacted at 120 °C for 24 h.b Isolated yield based on diphenyl diselenide.c TBHP in water.d 110 °C.e 100 °C.f 16 h.g 0.5 mL of 4-methoxybenzaldehyde was used.h Reaction was carried out under microwave conditions for 30 min. (TBHP = tert-butyl hydroperoxide, TBPB = tert-butyl peroxybenzoate, BPO = benzoyl peroxide, AcOOH = peracetic acid, DTBP = di-tert-butyl peroxide). | ||
| 1c | TBHP (4.0) | Trace |
| 2 | TBPB (4.0) | 64 |
| 3 | BPO (4.0) | Trace |
| 4 | K2S2O8 (4.0) | Trace |
| 5 | AcOOH (4.0) | N.R. |
| 6 | m-CPBA (4.0) | N.R. |
| 7 | H2O2 (4.0) | Trace |
| 8 | DTBP (4.0) | 93 |
| 9 | DTBP (3.0) | 41 |
| 10 | DTBP (2.0) | 21 |
| 11d | DTBP (4.0) | 31 |
| 12e | DTBP (4.0) | 16 |
| 13f | DTBP (4.0) | 68 |
| 14g | DTBP (4.0) | 59 |
| 15h | DTBP (4.0) | 68 |
With the optimized reaction conditions in hand, we then demonstrated the scope of this novel system for a variety of substrates. As shown in Table 2, a wide range of diaryl diselenides were smoothly coupled with aldehydes, giving the corresponding selenoesters in good to excellent yields. Diaryl diselenides bearing electron-donating and electron-withdrawing groups were successfully reacted with substituted aryl aldehydes. Remarkably, this system shows good functional group comparability, functional groups including bromo (Table 2, entries 1–5), trifluoromethyl (Table 2, entries 6–11), methoxy (Table 2, entries 16 and 17), amine (Table 2, entries 12 and 13) were all tolerated by the reaction conditions employed. Moreover, sterically demanding ortho-substituted aryl aldehydes underwent the C–Se bond formation with diselenides to provide the targets in good yields (Table 2, entry 2). Notably, heteroaromatic aldehydes such as thiophene-2-carboxaldehyde (Table 2, entries 5, 7, 14 and 16) and furan-containing aldehyde (Table 2, entries 8 and 17) are coupled with different diaryl diselenides bearing electron-donating and electron-withdrawing groups, provided the resulting selenoesters in good to excellent yields.
| Entry | Product | Yieldb (%) |
|---|---|---|
| a Reaction conditions unless otherwise stated: aldehyde (1.0 mL), diaryl diselenide (0.25 mmol) and DTBP (2.0 mmol) were reacted at 120 °C for 24 h using Schlenk tube.b Isolated yields based on diselenides.c Reactions were carried out using sealed tube. | ||
| 1 |  |
94 |
| 2 |  |
88 |
| 3 |  |
76 |
| 4 |  |
62 |
| 5 |  |
88 |
| 6 |  |
78 |
| 7 | 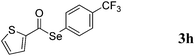 |
86 |
| 8 |  |
74 |
| 9 | 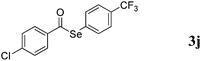 |
77 |
| 10 |  |
59 (90)c |
| 11 |  |
52 (91)c |
| 12 |  |
79 |
| 13 |  |
60 |
| 14 | 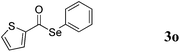 |
65 |
| 15 |  |
92 |
| 16 |  |
88 |
| 17 | 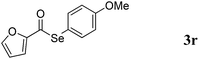 |
72 |
With the promising results in the coupling of aldehydes with diaryl diselenides, we next turned our attention to the use of dialkyl diselenides as the coupling partners in this system, the results are summarized in Table 3. A variety of aryl aldehydes bearing electron-withdrawing and electron-donating groups were successfully coupled with dialkyl diselenides, provided the corresponding selenoesters in moderate to excellent yields. Functional groups including chloro (Table 3, entry 8), trifluoromethyl (Table 3, entry 4), amine (Table 3, entries 1 and 2) were tolerated by the reaction conditions. Importantly, thiophene-containing alkyl selenoesters could also be formed in a 92% yield when the reaction was carried out by using 2-thiophenecarboxaldehyde as the coupling partner (Table 3, entry 3).
| Entry | Product | Yieldb (%) |
|---|---|---|
| a Reaction conditions unless otherwise stated: aldehyde (1.0 mL), dialkyl diselenide (0.25 mmol) and DTBP (2.0 mmol) were reacted at 120 °C for 24 h using sealed tube.b Isolated yields based on diselenides.c Reactions were carried out using Schlenk tube. | ||
| 1 |  |
91 (38)c |
| 2 |  |
84 |
| 3 |  |
92 |
| 4 |  |
67 |
| 5 |  |
78 |
| 6 | 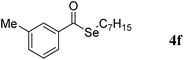 |
69 |
| 7 |  |
58 |
| 8 |  |
51 |
| 9 |  |
25 |
A potential mechanism for DTBP-promoted C–Se coupling reactions of aldehydes with diselenides is depicted in Scheme 1. The DTBP under heating can generate t-BuO radical which can react with aldehyde and diselenide to generate aldehydic radical A and selenide radical B respectively. The coupling of radical A and B can provide the selenoester.
Experimental section
General information
All chemicals were purchased from commercial suppliers and used without further purification. NMR spectra were recorded on a Varian Unity Inova-600 or a Varian Mercury-400 instrument using CDCl3 as solvent. Chemical shifts are reported in parts per million (ppm) and referenced to the residual solvent resonance. Coupling constant (J) are reported in hertz (Hz). Standard abbreviations indicating multiplicity were used as follows: s = singlet, d = doublet, t = triplet, dd = double doublet, q = quartet, m = multiplet. High resolution mass spectra (HRMS) were performed on an electron ionization time-of-flight (EI-TOF) mass spectrometer at the National Chung Hsing University.General procedure for Table 1
A Schlenk tube equipped with a magnetic stirrer bar was charged with diphenyl diselenide (78.8 mg, 0.25 mmol), 4-methoxybenzaldehyde (1.0 mL) and oxidant (2.0 mmol) under a nitrogen-filled balloon and heated at 120 °C for 24 h in an oil bath. After the reaction was complete (monitored by TLC), the reaction mixture was cooled to ambient temperature. The resulting residue was purified by column chromatography (SiO2, hexanes–EtOAc: 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3a.
1) to provide 3a.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), provided 3a as a white solid (135 mg, 93% yield). M.P.: 59–60 °C; 1H NMR (400 MHz, CDCl3): δ 3.83 (s, 3H), 6.93 (dd, J = 2.0 & 6.8 Hz, 2H), 7.40 (dd, J = 2.0 & 4.8 Hz, 3H), 7.57–7.59 (m, 2H), 7.90 (dd, J = 2.0 & 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 55.4, 114.0, 125.8, 128.8, 129.2, 129.5, 131.1, 136.3, 164.0, 191.2.
1), provided 3a as a white solid (135 mg, 93% yield). M.P.: 59–60 °C; 1H NMR (400 MHz, CDCl3): δ 3.83 (s, 3H), 6.93 (dd, J = 2.0 & 6.8 Hz, 2H), 7.40 (dd, J = 2.0 & 4.8 Hz, 3H), 7.57–7.59 (m, 2H), 7.90 (dd, J = 2.0 & 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 55.4, 114.0, 125.8, 128.8, 129.2, 129.5, 131.1, 136.3, 164.0, 191.2.General procedure for Table 2
A Schlenk tube equipped with a magnetic stirrer bar was charged with diselenide (0.25 mmol), aldehyde (1.0 mL) and DTBP (2.0 mmol) under a nitrogen-filled balloon and heated at 120 °C for 24 h in an oil bath. After the reaction was complete (monitored by TLC), the reaction mixture was cooled to ambient temperature. The resulting residue was purified by column chromatography (SiO2, hexanes–EtOAc: 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3.
1) to provide 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3b as a white solid (166 mg, 94% yield). M.P.: 64–65 °C; 1H NMR (400 MHz, CDCl3): δ 2.40 (s, 3H), 7.35 (d, J = 7.2 Hz, 1H), 7.39–7.41 (m, 3H), 7.50–7.52 (m, 2H), 7.69–7.71 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 21.2, 123.6, 124.5, 124.6, 127.6, 128.7, 132.3, 134.7, 137.7, 138.0, 138.8, 192.5; HRMS-EI calcd for C14H11BrOSe: 353.9158, found: 353.9162.
1) to provide 3b as a white solid (166 mg, 94% yield). M.P.: 64–65 °C; 1H NMR (400 MHz, CDCl3): δ 2.40 (s, 3H), 7.35 (d, J = 7.2 Hz, 1H), 7.39–7.41 (m, 3H), 7.50–7.52 (m, 2H), 7.69–7.71 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 21.2, 123.6, 124.5, 124.6, 127.6, 128.7, 132.3, 134.7, 137.7, 138.0, 138.8, 192.5; HRMS-EI calcd for C14H11BrOSe: 353.9158, found: 353.9162.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3c as a white solid (156 mg, 88% yield). M.P.: 87–88 °C; 1H NMR (400 MHz, CDCl3): δ 2.46 (s, 6H), 7.25 (d, J = 7.6 Hz, 1H), 7.32 (d, J = 7.6 Hz, 1H), 7.39–7.42 (m, 3H), 7.52–7.54 (m, 2H), 7.87 (d, J = 7.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 20.8, 123.7, 125.7, 126.0, 128.8, 131.9, 132.42, 132.48, 136.6, 137.6, 137.8, 194.2; HRMS-EI calcd for C14H11BrOSe: 353.9158, found: 353.9156.
1) to provide 3c as a white solid (156 mg, 88% yield). M.P.: 87–88 °C; 1H NMR (400 MHz, CDCl3): δ 2.46 (s, 6H), 7.25 (d, J = 7.6 Hz, 1H), 7.32 (d, J = 7.6 Hz, 1H), 7.39–7.42 (m, 3H), 7.52–7.54 (m, 2H), 7.87 (d, J = 7.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 20.8, 123.7, 125.7, 126.0, 128.8, 131.9, 132.42, 132.48, 136.6, 137.6, 137.8, 194.2; HRMS-EI calcd for C14H11BrOSe: 353.9158, found: 353.9156.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3e as a white solid (115 mg, 62% yield). M.P.: 81–82 °C; 1H NMR (400 MHz, CDCl3): δ 3.86 (s, 3H), 6.94 (d, J = 8.8 Hz, 2H), 7.43 (d, J = 8.8 Hz, 2H), 7.51 (d, J = 8.4 Hz, 2H), 7.87 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 59.5, 114.1, 123.6, 124.7, 129.6, 130.8, 132.3, 137.8, 164.2, 190.4; HRMS-EI calcd for C14H11BrO2Se: 369.9108, found: 369.9110.
1) to provide 3e as a white solid (115 mg, 62% yield). M.P.: 81–82 °C; 1H NMR (400 MHz, CDCl3): δ 3.86 (s, 3H), 6.94 (d, J = 8.8 Hz, 2H), 7.43 (d, J = 8.8 Hz, 2H), 7.51 (d, J = 8.4 Hz, 2H), 7.87 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 59.5, 114.1, 123.6, 124.7, 129.6, 130.8, 132.3, 137.8, 164.2, 190.4; HRMS-EI calcd for C14H11BrO2Se: 369.9108, found: 369.9110.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3f as a white solid (152 mg, 88% yield). M.P.: 77–78 °C; 1H NMR (400 MHz, CDCl3): δ 7.14 (dd, J = 0.8 & 4.0 Hz, 1H), 7.43–7.45 (m, 2H), 7.50–7.52 (m, 2H), 7.69 (dd, J = 1.2 & 4.8 Hz, 1H), 7.84 (dd, J = 1.2 & 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 123.8, 124.2, 128.0, 132.1, 132.4, 133.9, 137.6, 142.5, 182.6; HRMS-EI calcd for C11H7BrOSSe: 345.8566, found: 345.8564.
1) to provide 3f as a white solid (152 mg, 88% yield). M.P.: 77–78 °C; 1H NMR (400 MHz, CDCl3): δ 7.14 (dd, J = 0.8 & 4.0 Hz, 1H), 7.43–7.45 (m, 2H), 7.50–7.52 (m, 2H), 7.69 (dd, J = 1.2 & 4.8 Hz, 1H), 7.84 (dd, J = 1.2 & 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 123.8, 124.2, 128.0, 132.1, 132.4, 133.9, 137.6, 142.5, 182.6; HRMS-EI calcd for C11H7BrOSSe: 345.8566, found: 345.8564.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3g as a white solid (127 mg, 78% yield). M.P.: 96–97 °C; 1H NMR (400 MHz, CDCl3): δ 7.46–7.50 (m, 2H), 7.60–7.72 (m, 5H), 7.90–7.92 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 123.9 (d, J = 271.3 Hz), 125.9 (d, J = 3.7 Hz), 127.3, 129.0, 130.4, 131.0 (d, J = 32.8 Hz), 134.2, 136.4, 138.0, 192.0; 19F NMR (376 MHz, CDCl3): δ = −64.2 (s); HRMS-EI calcd for C14H9F3OSe: 329.9771, found: 329.9776.
1) to provide 3g as a white solid (127 mg, 78% yield). M.P.: 96–97 °C; 1H NMR (400 MHz, CDCl3): δ 7.46–7.50 (m, 2H), 7.60–7.72 (m, 5H), 7.90–7.92 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 123.9 (d, J = 271.3 Hz), 125.9 (d, J = 3.7 Hz), 127.3, 129.0, 130.4, 131.0 (d, J = 32.8 Hz), 134.2, 136.4, 138.0, 192.0; 19F NMR (376 MHz, CDCl3): δ = −64.2 (s); HRMS-EI calcd for C14H9F3OSe: 329.9771, found: 329.9776.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3h as yellow solid (144 mg, 86% yield). M.P.: 71–72 °C; 1H NMR (400 MHz, CDCl3): δ 7.14–7.17 (m, 1H), 7.62–7.70 (m, 2H), 7.71–7.72 (m, 3H), 7.85–7.87 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 123.8 (d, J = 270.3 Hz), 125.9 (d, J = 3.7 Hz), 128.1, 130.1, 131.0 (d, J = 32.8 Hz), 132.3, 134.1, 136.2, 142.4, 182.0; 19F NMR (376 MHz, CDCl3): δ = −64.2 (s). HRMS-EI calcd for C12H7F3OSSe: 335.9335, found: 335.9340.
1) to provide 3h as yellow solid (144 mg, 86% yield). M.P.: 71–72 °C; 1H NMR (400 MHz, CDCl3): δ 7.14–7.17 (m, 1H), 7.62–7.70 (m, 2H), 7.71–7.72 (m, 3H), 7.85–7.87 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 123.8 (d, J = 270.3 Hz), 125.9 (d, J = 3.7 Hz), 128.1, 130.1, 131.0 (d, J = 32.8 Hz), 132.3, 134.1, 136.2, 142.4, 182.0; 19F NMR (376 MHz, CDCl3): δ = −64.2 (s). HRMS-EI calcd for C12H7F3OSSe: 335.9335, found: 335.9340.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3i as a yellow oil (118 mg, 74% yield). 1H NMR (400 MHz, CDCl3): δ 6.60 (dd, J = 1.6, 3.6 Hz, 1H), 7.23 (dd, J = 0.8, 3.6 Hz, 1H), 7.63–7.72 (m, 5H); 13C NMR (100 MHz, CDCl3): δ 113.0, 115.7, 123.8 (d, J = 270.3 Hz), 125.9 (d, J = 3.7 Hz), 129.4, 131.0 (d, J = 32.8 Hz), 136.3, 146.9, 151.2, 179.4; 19F NMR (376 MHz, CDCl3): δ = −64.2 (s); HRMS-EI calcd for C12H7F3O2Se: 319.9563, found: 319.9561.
1) to provide 3i as a yellow oil (118 mg, 74% yield). 1H NMR (400 MHz, CDCl3): δ 6.60 (dd, J = 1.6, 3.6 Hz, 1H), 7.23 (dd, J = 0.8, 3.6 Hz, 1H), 7.63–7.72 (m, 5H); 13C NMR (100 MHz, CDCl3): δ 113.0, 115.7, 123.8 (d, J = 270.3 Hz), 125.9 (d, J = 3.7 Hz), 129.4, 131.0 (d, J = 32.8 Hz), 136.3, 146.9, 151.2, 179.4; 19F NMR (376 MHz, CDCl3): δ = −64.2 (s); HRMS-EI calcd for C12H7F3O2Se: 319.9563, found: 319.9561.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3k as a white solid (106 mg, 59% yield). M.P.: 90–91 °C; 1H NMR (400 MHz, CDCl3): δ 3.86 (s, 3H), 6.95 (dd, J = 2.0, 7.2 Hz, 2H), 7.63 (d, J = 8.0 Hz, 2H), 7.70 (d, J = 8.0 Hz, 2H), 7.89 (dd, J = 2.0, 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 55.5, 114.2, 123.9 (d, J = 270.3 Hz), 125.8 (d, J = 3.6 Hz), 129.7, 130.6, 130.7, 131.0 (d, J = 23.7 Hz), 136.4, 164.4, 189.8; 19F NMR (376 MHz, CDCl3): δ = −64.1 (s). HRMS-EI calcd for C15H11F3O2Se: 359.9876, found: 359.9872.
1) to provide 3k as a white solid (106 mg, 59% yield). M.P.: 90–91 °C; 1H NMR (400 MHz, CDCl3): δ 3.86 (s, 3H), 6.95 (dd, J = 2.0, 7.2 Hz, 2H), 7.63 (d, J = 8.0 Hz, 2H), 7.70 (d, J = 8.0 Hz, 2H), 7.89 (dd, J = 2.0, 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 55.5, 114.2, 123.9 (d, J = 270.3 Hz), 125.8 (d, J = 3.6 Hz), 129.7, 130.6, 130.7, 131.0 (d, J = 23.7 Hz), 136.4, 164.4, 189.8; 19F NMR (376 MHz, CDCl3): δ = −64.1 (s). HRMS-EI calcd for C15H11F3O2Se: 359.9876, found: 359.9872.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), provided 3m as a yellow solid (131 mg, 79% yield). M.P.: 104–106 °C; 1H NMR (400 MHz, CDCl3): δ 1.09 (t, J = 7.2 Hz, 6H), 3.30 (q, J = 7.2 Hz, 4H), 6.52 (d, J = 9.2 Hz, 2H), 7.28–7.29 (m, 2H), 7.50–7.52 (m, 3H), 7.71 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 12.3, 44.5, 110.2, 124.8, 126.5, 128.4, 128.9, 130.0, 136.4, 151.8, 189.2; HRMS-EI calcd for C17H19NOSe: 333.0632, found: 333.0640.
1), provided 3m as a yellow solid (131 mg, 79% yield). M.P.: 104–106 °C; 1H NMR (400 MHz, CDCl3): δ 1.09 (t, J = 7.2 Hz, 6H), 3.30 (q, J = 7.2 Hz, 4H), 6.52 (d, J = 9.2 Hz, 2H), 7.28–7.29 (m, 2H), 7.50–7.52 (m, 3H), 7.71 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 12.3, 44.5, 110.2, 124.8, 126.5, 128.4, 128.9, 130.0, 136.4, 151.8, 189.2; HRMS-EI calcd for C17H19NOSe: 333.0632, found: 333.0640.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), provided 3n as a yellow solid (91 mg, 60% yield). M.P.: 162–163 °C; 1H NMR (400 MHz, CDCl3): δ 2.97 (s, 6H), 6.55 (d, J = 9.2 Hz, 2H), 7.30–7.32 (m, 3H), 7.50–7.53 (m, 2H), 7.74 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 39.9, 110.7, 125.5, 126.4, 128.5, 129.0, 129.7, 136.4, 153.9, 189.7; HRMS-EI calcd for C15H15NOSe: 305.0319, found: 305.0310.
1), provided 3n as a yellow solid (91 mg, 60% yield). M.P.: 162–163 °C; 1H NMR (400 MHz, CDCl3): δ 2.97 (s, 6H), 6.55 (d, J = 9.2 Hz, 2H), 7.30–7.32 (m, 3H), 7.50–7.53 (m, 2H), 7.74 (d, J = 8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 39.9, 110.7, 125.5, 126.4, 128.5, 129.0, 129.7, 136.4, 153.9, 189.7; HRMS-EI calcd for C15H15NOSe: 305.0319, found: 305.0310.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), provided 3o as a brown oil (86 mg, 65% yield). 1H NMR (400 MHz, CDCl3): δ 7.14 (t, J = 4.8 Hz, 1H), 7.39–7.41 (m, 3H), 7.58–7.60 (m, 2H), 7.67 (d, J = 5.2 Hz, 1H), 7.86 (d, J = 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 125.4, 127.9, 129.1, 129.3, 131.9, 133.16, 136.1, 142.9, 183.4.
1), provided 3o as a brown oil (86 mg, 65% yield). 1H NMR (400 MHz, CDCl3): δ 7.14 (t, J = 4.8 Hz, 1H), 7.39–7.41 (m, 3H), 7.58–7.60 (m, 2H), 7.67 (d, J = 5.2 Hz, 1H), 7.86 (d, J = 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 125.4, 127.9, 129.1, 129.3, 131.9, 133.16, 136.1, 142.9, 183.4.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3q as a brown solid (131 mg, 88% yield). M.P.: 101–102 °C; 1H NMR (400 MHz, CDCl3): δ 3.81 (s, 3H), 6.91–6.94 (m, 2H), 7.12–7.14 (m, 1H), 7.47–7.49 (m, 2H), 7.66 (dd, J = 1.2, 4.8 Hz, 1H), 7.85 (dd, J = 1.2, 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 55.2, 115.0, 115.7, 127.9, 131.8, 133.4, 137.7, 143.0, 160.4, 184.3; HRMS-EI calcd for C12H10O2SSe: 297.9567, found: 297.9572.
1) to provide 3q as a brown solid (131 mg, 88% yield). M.P.: 101–102 °C; 1H NMR (400 MHz, CDCl3): δ 3.81 (s, 3H), 6.91–6.94 (m, 2H), 7.12–7.14 (m, 1H), 7.47–7.49 (m, 2H), 7.66 (dd, J = 1.2, 4.8 Hz, 1H), 7.85 (dd, J = 1.2, 4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 55.2, 115.0, 115.7, 127.9, 131.8, 133.4, 137.7, 143.0, 160.4, 184.3; HRMS-EI calcd for C12H10O2SSe: 297.9567, found: 297.9572.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 3r as a brown solid (101 mg, 72% yield). M.P.: 51–52 °C; 1H NMR (400 MHz, CDCl3): δ 3.82 (s, 3H), 6.56 (dd, J = 1.6, 3.6 Hz, 1H), 6.92–6.95 (m, 2H), 7.19 (d, J = 3.6 Hz, 1H), 7.47 (dd, J = 2.0, 6.8 Hz, 2H), 7.62 (d, J = 1.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 55.21, 112.7, 114.9, 115.0, 115.3, 137.8, 146.5, 151.7, 160.4, 181.6; HRMS-EI calcd for C12H10O3Se: 281.9795, found: 281.9801.
1) to provide 3r as a brown solid (101 mg, 72% yield). M.P.: 51–52 °C; 1H NMR (400 MHz, CDCl3): δ 3.82 (s, 3H), 6.56 (dd, J = 1.6, 3.6 Hz, 1H), 6.92–6.95 (m, 2H), 7.19 (d, J = 3.6 Hz, 1H), 7.47 (dd, J = 2.0, 6.8 Hz, 2H), 7.62 (d, J = 1.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 55.21, 112.7, 114.9, 115.0, 115.3, 137.8, 146.5, 151.7, 160.4, 181.6; HRMS-EI calcd for C12H10O3Se: 281.9795, found: 281.9801.General procedure for Table 3
A sealed tube equipped with a magnetic stirrer bar was charged with diselenide (0.25 mmol), aldehyde (1.0 mL) and DTBP (2.0 mmol) under a nitrogen-filled balloon and heated at 120 °C for 24 h in an oil bath. After the reaction was complete (monitored by TLC), the reaction mixture was cooled to ambient temperature. The resulting residue was purified by column chromatography (SiO2, hexanes–EtOAc: 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 4.
1) to provide 4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), provided 4a as a white solid (123 mg, 91% yield). M.P.: 79–81 °C; 1H NMR (400 MHz, CDCl3): δ 1.17 (t, J = 7.2 Hz, 6H), 2.31 (s, 3H), 3.38 (q, J = 7.2 Hz, 4H), 6.58 (d, J = 9.2 Hz, 2H), 7.78 (d, J = 9.2 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 4.2, 12.3, 44.4, 110.1, 125.6, 129.6, 151.4, 191.0; HRMS-EI calcd for C12H17NOSe: 271.0475, found: 271.0472.
1), provided 4a as a white solid (123 mg, 91% yield). M.P.: 79–81 °C; 1H NMR (400 MHz, CDCl3): δ 1.17 (t, J = 7.2 Hz, 6H), 2.31 (s, 3H), 3.38 (q, J = 7.2 Hz, 4H), 6.58 (d, J = 9.2 Hz, 2H), 7.78 (d, J = 9.2 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 4.2, 12.3, 44.4, 110.1, 125.6, 129.6, 151.4, 191.0; HRMS-EI calcd for C12H17NOSe: 271.0475, found: 271.0472.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), provided 4b as a white solid (102 mg, 84% yield). M.P.: 87–89 °C; 1H NMR (400 MHz, CDCl3): δ 2.31 (s, 3H), 3.01 (s, 6H), 6.59 (d, J = 9.2 Hz, 2H), 7.79 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 4.3, 39.9, 110.5, 126.4, 129.2, 153.7, 191.4; HRMS-EI calcd for C10H13NOSe: 243.0162, found: 243.0160.
1), provided 4b as a white solid (102 mg, 84% yield). M.P.: 87–89 °C; 1H NMR (400 MHz, CDCl3): δ 2.31 (s, 3H), 3.01 (s, 6H), 6.59 (d, J = 9.2 Hz, 2H), 7.79 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 4.3, 39.9, 110.5, 126.4, 129.2, 153.7, 191.4; HRMS-EI calcd for C10H13NOSe: 243.0162, found: 243.0160.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide 4i as a yellow oil (38.2 mg, 25% yield). 1H NMR (400 MHz, CDCl3): δ 3.85 (s, 3H), 4.32 (s, 2H), 6.90 (dd, J = 2.0, 6.8 Hz, 2H), 7.20–7.37 (m, 5H), 7.86 (dd, J = 2.0, 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 28.8, 55.5, 113.9, 126.8, 128.5, 128.9, 129.5, 131.6, 139.2, 164.0, 192.4; HRMS-EI calcd for C15H14O2Se: 306.0159, found: 306.0153.
1) to provide 4i as a yellow oil (38.2 mg, 25% yield). 1H NMR (400 MHz, CDCl3): δ 3.85 (s, 3H), 4.32 (s, 2H), 6.90 (dd, J = 2.0, 6.8 Hz, 2H), 7.20–7.37 (m, 5H), 7.86 (dd, J = 2.0, 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 28.8, 55.5, 113.9, 126.8, 128.5, 128.9, 129.5, 131.6, 139.2, 164.0, 192.4; HRMS-EI calcd for C15H14O2Se: 306.0159, found: 306.0153.Conclusions
In conclusion, we have developed a general and efficient approach for the preparation of selenoesters using DTBP as an oxidant under metal-free and solvent-free conditions. This system shows good functional group compatibility, giving selenoesters in good to excellent yields.Acknowledgements
The National Science Council, Taiwan (NSC 101-2113-M-005-008-MY3), the National Chung Hsing University and the Center of Nanoscience and Nanotechnology (NCHU) are gratefully acknowledged for financial support. We also thank Prof. Fung-E Hong (NCHU) for sharing his GC-MS instruments. C.F.L. is a Golden-Jade Fellow of Kenda Foundation, Taiwan.Notes and references
- (a) R. J. Shamberger, in Biochemistry of Selenium, Plenum Press, New York, 1983 Search PubMed; (b) D. L. Klayman and H. H. Gunter, in Organoselenium Compounds: Their Chemistry and Biology, Wiley-Interscience, New York, 1973 Search PubMed; (c) J. T. Rotruck, A. L. Pope, H. E. Ganther, A. B. Swanson, D. G. Hafeman and W. G. Hoekstra, Science, 1973, 179, 588 CAS; (d) L. Flohe, E. A. Gunzler and H. H. Schock, FEBS Lett., 1973, 32, 132 CrossRef CAS; (e) L. Flohe, J. R. Andreesen, R. Brigelius-Flohe, M. Maiorino and F. Ursini, IUBMB Life, 2000, 49, 411 CrossRef CAS PubMed; (f) C. Jacob, G. I. Giles, N. M. Giles and H. Sies, Angew. Chem., Int. Ed., 2003, 42, 4742 CrossRef CAS PubMed; (g) B. K. Sarma and G. Mugesh, Org. Biomol. Chem., 2008, 6, 965 RSC; (h) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255 CrossRef CAS PubMed; (i) G. Mugesh, W. W. du Mont and H. Sies, Chem. Rev., 2001, 101, 2125 CrossRef CAS PubMed; (j) G. Mugesh and H. Singh, Chem. Soc. Rev., 2000, 29, 347 RSC.
- (a) S.-i. Fukuzawa and K. Tsudsuki, Tetrahedron: Asymmetry, 1995, 6, 1039 CrossRef CAS; (b) T. Wirth, Tetrahedron Lett., 1995, 36, 7849 CrossRef CAS; (c) A. L. Braga, F. Vargas, J. A. Sehnem and R. C. Braga, J. Org. Chem., 2005, 70, 9021 CrossRef CAS PubMed; (d) M. Zielinska-Blajet, R. Siedlecka and J. Skarzewski, Tetrahedron: Asymmetry, 2007, 18, 131 CrossRef CAS PubMed; (e) R. S. Schwab, L. C. Soares, L. Dornelles, O. E. D. Rodrigues, M. W. Paixao, M. Godoi and A. L. Braga, Eur. J. Org. Chem., 2010, 3574 CrossRef CAS PubMed; (f) F. Vargas, J. A. Sehnem, F. Z. Galetto and A. L. Braga, Tetrahedron, 2008, 64, 392 CrossRef CAS PubMed; (g) T. Wirth, K. J. Kulicke and G. Fragale, Helv. Chim. Acta, 1996, 79, 1957 CrossRef CAS PubMed; (h) E. Wojaczynska and J. Skazewski, Tetrahedron: Asymmetry, 2008, 19, 593 CrossRef CAS PubMed.
- For reviews, see: (a) T. Wirth, Tetrahedron, 1999, 55, 1 CrossRef CAS; (b) T. Wirth, Angew. Chem., Int. Ed., 2000, 39, 3740 CrossRef CAS; (c) A. L. Braga, D. S. Ludtke, F. Vargas and R. C. Braga, Synlett, 2006, 1453 CrossRef CAS PubMed; (d) A. L. Braga, D. S. Ludtke, F. Vargas and R. C. Braga, Curr. Org. Chem., 2006, 10, 1921 CrossRef CAS; (e) M. Godoi, M. W. Paixao and A. L. Braga, Dalton Trans., 2011, 40, 11347 RSC.
- (a) A. L. Braga, T. Barcellos, M. W. Paixao, A. M. Deobald, M. Godoi, H. A. Stefani, R. Cella and A. Sharma, Organometallics, 2008, 27, 4009 CrossRef CAS; (b) G. Perin, E. J. Lenardao, R. G. Jacob and R. B. Panatieri, Chem. Rev., 2009, 109, 1277 CrossRef CAS PubMed.
- (a) Topics in Current Chemistry in Organoselenium Chemistry, ed. T. Wirth, Springer, Heidelberg, 2000, p. 208 Search PubMed; (b) A. Krief, in Comprehensive Organometallic Chemistry II, ed. E. V. Abel, F. G. A. Stone and G. Wilkinson, Pergamon Press, New York, 1995, vol. 11, ch. 13, p. 515 Search PubMed; (c) C. Paulmier, in Selenium Reagents and Intermediates in Organic Synthesis, Organic Chemistry Series 4, ed. J. E. Baldwin, Pergamon Press, Oxford, 1986 Search PubMed.
- (a) D. M. Freudendahl, M. Iwaoka and T. Wirth, Eur. J. Org. Chem., 2010, 3934 CrossRef CAS PubMed; (b) L. Zhao, Z. Li and T. Wirth, Eur. J. Org. Chem., 2011, 176 CrossRef CAS PubMed; (c) S. A. Shahzad, C. Vivant and T. Wirth, Org. Lett., 2010, 12, 1364 CrossRef CAS PubMed; (d) A. L. Braga, G. Zeni, L. H. Andrade and C. C. Silveira, Synlett, 1997, 595 CrossRef CAS PubMed; (e) D. M. Browne, O. Niyomura and T. Wirth, Org. Lett., 2007, 9, 3169 CrossRef CAS PubMed; (f) M. Tiecco, L. Testaferri, M. Tingoli, L. Bagnoli and C. Santi, Synlett, 1993, 798 CrossRef CAS PubMed.
- (a) G. E. Keck and M. C. Grier, Synlett, 1999, 1657 CrossRef CAS PubMed; (b) C. Chen, D. Crich and A. Papadatos, J. Am. Chem. Soc., 1992, 114, 8313 CrossRef CAS; (c) D. L. Boger and R. J. Mathvink, J. Org. Chem., 1992, 57, 1429 CrossRef CAS; (d) T. Hiiro, Y. Morita, T. Inoue, N. Kambe, A. Ogawa, I. Ryuand and N. Sonoda, J. Am. Chem. Soc., 1990, 112, 455 CrossRef CAS; (e) A. F. Sviridov, M. S. Erolenko, D. V. Yashunky and N. K. Kochetkov, Tetrahedron Lett., 1983, 24, 4355 CrossRef CAS.
- (a) G. Heppke, J. Martens, K. Praefcke and H. Simon, Angew. Chem., Int. Ed. Engl., 1977, 16, 318 CrossRef PubMed; (b) J.-i. Yamada, H. Akutsu, H. Nishikawa and K. Kikuchi, Chem. Rev., 2004, 104, 5057 CrossRef CAS PubMed.
- (a) M. Inoue, S. Yamahita, Y. Ishihara and M. Hirama, Org. Lett., 2006, 8, 5805 CrossRef CAS PubMed; (b) S. F. Martin, K. X. Chen and C. T. Eary, Org. Lett., 1999, 1, 79 CrossRef CAS.
- (a) T. Inoue, T. Takeda, N. Kambe, A. Ogawa, I. Ryu and N. Sonoda, J. Org. Chem., 1994, 59, 5824 CrossRef CAS; (b) S.-I. Fujiwara, A. Asai, T. Shin-ike, N. Kambe and N. Sonoda, J. Org. Chem., 1998, 63, 1724 CrossRef CAS; (c) M. Tingoli, A. Temperini, L. Testaferri and M. Tiecco, Synlett, 1995, 1129 CrossRef CAS PubMed; (d) W. Dan, H. Deng, J. Chen, M. Liu, J. Ding and H. Wu, Tetrahedron, 2010, 66, 7384 CrossRef CAS PubMed; (e) Y. Nishiyama, K. Tokunaga, H. Kawamatsu and N. Sonoda, Tetrahedron Lett., 2002, 43, 1507 CrossRef CAS.
- (a) A. L. Braga, T. L. C. Martins, C. C. Silveira and O. E. D. Rodrigues, Tetrahedron, 2001, 57, 3297 CrossRef CAS; (b) M. Tiecco, L. Testaferri, A. Temperini, L. Bagnoli, F. Marini, C. Santi and R. Terlizzi, Eur. J. Org. Chem., 2004, 3447 CrossRef CAS PubMed.
- (a) C. C. Silveira, A. L. Braga and E. L. Larghi, Organometallics, 1999, 18, 5183 CrossRef CAS; (b) A. Capperucci, A. DeglInnocenti and C. Tiberi, Synlett, 2011, 2248 CrossRef CAS PubMed; (c) I. P. Beletskaya, A. S. Sigeev, A. S. Peregudov and P. V. Petrovskii, Russ. J. Org. Chem., 2001, 37, 1703 CrossRef CAS; (d) Y. Nishiyama, H. Kawamatsu, S. Funato, K. Tokunaga and N. Sonoda, J. Org. Chem., 2003, 68, 3599 CrossRef CAS PubMed; (e) G. Marin, A. L. Braga, A. S. Rosa, F. Z. Galetto, R. A. Burrow, H. Gallardo and M. W. Paixao, Tetrahedron, 2009, 65, 4614 CrossRef CAS PubMed; (f) B. C. Ranu and T. Mandal, J. Org. Chem., 2004, 69, 5793 CrossRef CAS PubMed; (g) K. Ren, M. Wang, P. Liu and L. Wang, Synthesis, 2010, 1078 CAS.
- (a) Y. Nishiyama, K. Tokunaga, H. Kawamatsu and N. Sonoda, Tetrahedron Lett., 2002, 43, 1507 CrossRef CAS; (b) R. Chena and Y. Zhang, Synth. Commun., 2000, 30, 1331 CrossRef; (c) G. Marin, A. L. Braga, A. S. Rosa, F. Z. Galetto, R. A. Burrowa, H. Gallardo and M. W. Paixao, Tetrahedron, 2009, 65, 4614 CrossRef CAS PubMed; (d) B. C. Ranu, T. Mandal and S. Samanta, Org. Lett., 2003, 5, 1439 CrossRef CAS PubMed; (e) K. Ajiki, M. Hirano and K. Tanaka, Org. Lett., 2005, 7, 4193 CrossRef CAS PubMed; (f) D. Singh, S. Narayanaperumal, K. Gul, M. Godoi, O. E. D. Rodrigues and A. L. Braga, Green Chem., 2010, 12, 957 RSC; (g) M. Godoi, E. W. Ricardo, G. V. Botteselle, F. Z. Galetto, J. B. Azeredo and A. L. Braga, Green Chem., 2012, 14, 456 RSC; (h) T. Nishino, M. Okada, T. Kuroki, T. Watanabe, Y. Nishiyama and N. Sonoda, J. Org. Chem., 2002, 67, 8696 CrossRef CAS PubMed.
- (a) O. A. Wallner and K. J. Szabo, J. Org. Chem., 2005, 70, 9215 CrossRef CAS PubMed; (b) C.-L. Yi, Y.-T. Huang and C.-F. Lee, Green Chem., 2013, 15, 2476 RSC.
- (a) J.-W. Zeng, Y.-C. Liu, P.-A. Hsiech, Y.-T. Huang, C.-L. Yi, S. S. Badsara and C.-F. Lee, Green Chem., 2014, 16, 2644 RSC; (b) C.-L. Sun, H. Li, D.-G. Yu, M. Yu, X. Zhou, X.-Y. Lu, K. Huang, S.-F. Zheng, B.-J. Li and Z.-J. Shi, Nat. Chem., 2010, 2, 1044 CrossRef CAS PubMed; (c) W. Liu, H. Cao, H. Zhang, H. Zhang, K. H. Chung, C. He, H. Wang, F. Y. Kwong and A. Lei, J. Am. Chem. Soc., 2010, 132, 16737 CrossRef CAS PubMed.
- During the preparation of this manuscript, one example of DTBP-promoted coupling of aldehydes with diselenides in organic solvent was reported by Sun's group, however, the substrates are very limited to diaryl diselenide (diphenyl diselenide is the only starting material in that work). See: C. Hea, X. Qian and P. Sun, Org. Bio. Chem., 2014, 12, 6072 RSC.
- (a) S. Banerjee, L. Adak and B. C. Ranu, Tetrahedron Lett., 2012, 53, 2149 CrossRef CAS PubMed; (b) G. P. Mullen, N. P. Luthra, R. B. Dunlap and J. D. Odom, J. Org. Chem., 1985, 50, 811 CrossRef CAS; (c) Y. Nishiyama, K. Tokunaga, H. Kawamatsu and N. Sonoda, Tetrahedron Lett., 2002, 43, 1507 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR spectra of compounds 3a–r and 4a–i. See DOI: 10.1039/c4ra07983c |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |