Biocompatible carboxymethylcellulose-g-poly(acrylic acid)/OMMT nanocomposite hydrogel for in vitro release of vitamin B12
Monalisha Boruaha,
Pronob Gogoia,
Ajay Kumar Manharb,
Momina Khannama,
Manabendra Mandalb and
Swapan Kumar Dolui*a
aDepartment of Chemical Sciences, Tezpur University, Napaam, Sonitpur, Assam, India-784028. E-mail: swapandolui@gmail.com; Tel: +91 9957198489
bDepartment of Molecular Biology and Biotechnology, Tezpur University, Napaam, Sonitpur, Assam, India-784028
First published on 19th August 2014
Abstract
The present work describes the preparation of a biocompatible nanocomposite hydrogel based on CMC-g-PAA and organo-MMT nanoclay by using methylene bis-acrylamide (MBA) as a cross-linker and potassium persulfate (KPS) as an initiator through radical graft polymerization. The nanocomposite hydrogels were characterized by using techniques such as FTIR, SEM and XRD analysis. The effects of various parameters on the swelling behaviour of the hydrogels were studied. The mechanical strength of the nanocomposite hydrogels was determined by dynamic mechanical analysis (DMA) and all the samples showed an increase in the storage modulus (G′) with an increase in cross-linker amount. The in vitro biocompatibility of the nanocomposite hydrogels showed that the presence of nanoclay in the nanocomposite hydrogel enhanced the in vitro blood compatibility. The vitamin B12 release mechanism has been studied during different time periods using a UV-visible spectrophotometer. The drug release kinetics revealed that release of vitamin B12 follows a non-Fickian diffusion mechanism.
1. Introduction
In recent years, stimuli-responsive polymeric hydrogels have attracted attention as “intelligent materials” because of their ability to mimic natural systems. They can sense a stimulus as a signal and give rise to volume changes in response to small changes in external stimuli, such as solvent composition,1 temperature,2 pH,3 salt concentration,4 and electrical pulses.5 Amongst the stimuli-responsive systems, pH-responsive hydrogels have been extensively studied in the biomedical field because the pH can be easily controlled and is applicable in both in vitro and in vivo conditions.6Carboxymethyl cellulose (CMC) is a type of cellulose derivative with carboxymethyl groups (–CH2–COOR) bound to some of the hydroxyl groups present on the cellulose backbone. It can be easily produced by the alkali-catalyzed reaction of cellulose with chloroacetic acid. The polar carboxyl groups provide solubility and chemical reactivity to cellulose and make it strongly hydrophilic in nature. However, the main disadvantage of natural polymer-based hydrogels is their poor mechanical properties due to extensive swelling. To get rid of this problem, attempts have been made to modify their structures by the incorporation of different nanofillers, grafting,7 developing interpenetrating polymer networks (IPNs),8 or by physical blending with other polymers.9 Poly(acrylic acid) is a hydrophilic polymer owing to the existence of hydrophilic –COOH groups and it has the capacity to absorb large amounts of water. For this reason, poly(acrylic acid) has been extensively used in controlled drug delivery systems.
Nanocomposites exhibit superior properties when compared to micro- and macro-composites. The strong interfacial interactions between the dispersed clay layers and the polymer matrix give rise to improved mechanical and thermal properties over those of the virgin polymer.10 Clays have been widely utilized in this field due to improved performance and low cost.11 Montmorillonite (MMT) is smective-type clay composed of an expandable 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type of aluminosilicate clay mineral, which has a layered structure and also a relatively high cation exchange capacity, large specific surface area, good swelling capacity and high platelet aspect ratio.12 It can expand considerably more than other clays due to the water penetration into the interlayer molecular spaces and concomitant adsorption. The organic cations can enter into the surface of clay through a cation exchange reaction; therefore, in nature, the clay can become organophillic.13
1 type of aluminosilicate clay mineral, which has a layered structure and also a relatively high cation exchange capacity, large specific surface area, good swelling capacity and high platelet aspect ratio.12 It can expand considerably more than other clays due to the water penetration into the interlayer molecular spaces and concomitant adsorption. The organic cations can enter into the surface of clay through a cation exchange reaction; therefore, in nature, the clay can become organophillic.13
Biocompatibility is a prime requirement for the development of functional biomaterials. It is governed mainly by the interface between foreign materials and host living cells/tissues. Therefore, the fundamental aspect of any biocompatibility evaluation is to gain knowledge of the effects of the polymers on human cells.14 Thus, the suitability of a hydrogel for biological applications can be confirmed by determining the potential toxicity of all materials, which have been used for the fabrication of the gel.
Nowadays, researchers are concentrating on developing natural polysaccharide based hydrogels due to their enhanced pH-responsive characteristics.15 Sadeghi et al. reported novel CMC-g-(PNVP-co-PAMPS) based hydrogels for the controlled release of metronidazole. They investigated the release behavior of metronidazole from this kind of responsive hydrogel by control of pH of the surrounding environment.16 Wang et al. synthesized a series of carboxymethyl cellulose/organic montmorillonite (CMC/OMMT) nanocomposites and studied their adsorption behavior for congo red dye from wastewater. The MMT clay was organically modified by using cetyl trimethylammonium bromide (CTAB) and it was found that the prepared nanocomposites exhibited improved thermal properties. The effect of various parameters such as CTAB content, weight ratio of CMC to OMMT, reaction time and reaction temperature on absorbtion behaviour of congo red dye was thoroughly investigated.17 Moreover, Irani et al. synthesized linear low-density polyethylene-g-poly(acrylic acid)/organo-montmorillonite superabsorbent hydrogel composites. They determined the optimum reaction conditions and effect of different parameters on the swelling behaviour of the superabsorbent hydrogels along with the equilibrium swelling. The gel strength of the nanocomposite hydrogel increased with the increase in OMMT content.18
To combine the advantages of synthetic and natural polymers and at the same time maintain the beneficial properties of natural polymers, we have prepared pH-sensitive hydrogels based on carboxymethylcellulose, acrylic acid and organically modified MMT nanoclay for the controlled release of vitamin B12, which is a water-soluble vitamin that plays a key role in the normal functioning of the brain and nervous system and in the formation of blood; therefore, vitamin B12 was chosen as a model drug. The present investigation deals with in vitro release studies on nanocomposite hydrogel formulations loaded with different amounts of vitamin B12. The drug release kinetics were studied and the effect of pH and cross-linker content on the release behavior of the prepared hydrogels has been determined.
2. Materials and methods
2.1 Materials
Acrylic acid monomer (Aldrich) was distilled under reduced pressure prior to use. Carboxymethylcellulose was supplied by Aldrich (Mw = 2,50,000). Potassium persulfate (KPS, analytical grade), methylene bis-acrylamide (MBA, chemically pure) as a cross-linking agent, montmorillonite nanoclay, and N,N,N′,N′-tetramethylethylene diamine were supplied by Aldrich (A.R. grade). Cetyltrimethylammonium bromide (CTAB) was supplied by G.S Chemical testing lab & allied industries, New Delhi. Sodium hydroxide and ethanol (A. R grade) were purchased from Merck, Mumbai. Vitamin B12 (C63H88CoN14O14P) was purchased from Merck, Mumbai (Mw = 1355.4 g mol−1).2.2 Preparation of organo-MMT nanoclay
The organophilic MMT-nanoclay was prepared by treating the pristine clay with cetyltrimethylammonium bromide (CTAB) as reported elsewhere.19 3 g of pristine clay was dispersed in 250 ml of deionized water under vigorous stirring. Then, a solution of CTAB (1.318 g in 100 ml of deionized water) was slowly added to the dispersion, under continuous stirring for 6 hours. The dispersion was filtered and the obtained product was thoroughly washed with deionized water. To determine the presence of released counterions, samples of the filtrate were taken at regular intervals and tested with a solution of 0.1 M silver. Washing was stopped only when the filtrate did not give a positive test to silver nitrate. The washed product was dried overnight under reduced pressure (vacuum) at 40 °C and finally ground in a mortar.2.3 Preparation of CMC-g-PAA/OMMT nanocomposite hydrogels
Carboxymethylcellulose-g-polyacrylic acid/OMMT nanocomposite hydrogels were prepared by free radical polymerization in distilled water. 2 g of CMC was dissolved in 30 ml of doubly distilled water and 1.5 ml of partially neutralized (60%) acrylic acid monomer was added to it. Moreover, a certain amount of OMMT nanoclay (0–20 wt%) was immersed in 5 ml of deionized water for 2 h and dispersed under ultrasonic vibration for 30 min. Both the solutions were mixed well and subsequently, methylene bis-acrylamide (0.05–0.25 wt%), potassium persulfate (0.1–1.0 wt%) and TEMED (0.05 ml) were added to the mixture under continuous stirring. Nitrogen was used to remove dissolved oxygen from the reactive solution. The reactive solution was first prepolymerized at 70 °C for 30 min under stirring, and then poured into a Petri dish quickly. The post polymerization was carried out at 65 °C for 15 h. When the reaction was completed, the nanocomposite hydrogel was cut into equal sized pieces (3 × 4 cm) and immersed in repeatedly changed deionized water for 72 h to remove the residual monomers. Tables 1–3 outlines the feed compositions of subsequently prepared hydrogels. Moreover, a proposed mechanism for the formation of nanocomposite hydrogel is shown in Fig. 1.| Recipe | CP-1 | CP-2 | CP-3 | CP-4 | CP-5 |
|---|---|---|---|---|---|
| a Tetramethylethylene diamine (ml) = 0.05 ml, organo-MMT nanoclay (wt%) = 10. | |||||
| Carboxymethyl cellulose (g) | 2 | 2 | 2 | 2 | 2 |
| Acrylic acid (ml) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Methylene bis-acrylamide (wt%) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Potassium persulfate (wt%) | 0.1 | 0.3 | 0.5 | 0.8 | 1.0 |
| Recipe | MP-1 | MP-2 | MP-3 | MP-4 | MP-5 |
|---|---|---|---|---|---|
| a Potassium persulfate (wt%) = 0.5, tetramethylethylene diamine (ml) = 0.05 ml and organo-MMT nanoclay (wt%) = 10. | |||||
| Carboxymethyl cellulose (g) | 2 | 2 | 2 | 2 | 2 |
| Acrylic acid (ml) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Methylene bis-acrylamide (wt%) | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 |
| ‘n’ value | 0.63 | 0.71 | 0.78 | 0.83 | 0.87 |
| Recipe | NP-1 | NP-2 | NP-3 | NP-4 | NP-5 |
|---|---|---|---|---|---|
| a Potassium persulfate (wt %) = 0.5, tetramethylethylene diamine (ml) = 0.05 ml. | |||||
| Carboxymethyl cellulose (g) | 2 | 2 | 2 | 2 | 2 |
| Acrylic acid (ml) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Methylene bis-acrylamide (wt%) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Organo-MMT nanoclay (wt%) | 0 | 5 | 10 | 15 | 20 |
| Gel fraction (%) | 74 | 78 | 84 | 89 | 93 |
2.4 Characterization of the nanocomposite hydrogel
Fourier transform infrared spectroscopy (FTIR) was used to record FTIR spectra by Impact 410, Nicolet, USA, using KBr pellets. Powder X-ray diffraction (XRD) data were collected on a Rigaku Miniflex X-ray diffractometer (Tokyo, Japan) with Cu Kα radiation (λ = 0.15418 nm) at 30 kV and 15 mA with a scanning rate of 0.05°s−1 in a 2θ range from 20° to 80°. The surface morphology of the composites was observed using a scanning electron microscope (SEM) (Model-JSM-6390LV, JEOL, Japan). The surface of the sample was coated with platinum before SEM analysis.The mechanical properties of the hydrogels were determined by dynamic mechanical analysis (DMA). The equilibrated swollen hydrogel samples were used for the measurement. The samples were cut in cylindrical shapes with about 6 mm diameter and 4 mm thickness. The samples were then loaded into a DMA (PerkinElmer DMA 8000), which applied a sinusoidally oscillating tensile strain, the resulting force waveform was measured, and using the input geometrical parameters the resulting stress waveform was determined. The frequency sweeping test experiments were performed under constant strain amplitude (1%) at a frequency range of 0.1 Hz to 10 Hz and storage moduli G′ and loss moduli G′′ were monitored as a function of frequency. In DMA, dynamic stress and dynamic strain are obtained describing the viscoelastic behavior of a material. The dynamic modulus, G*, is defined as follows:
| G* = G′ + iG′′ |
2.5 Swelling properties
To measure the degree of swelling of the hydrogels, dried samples were placed in buffer media of different pH values at room temperature until the hydrated gels reached a stable weight. The water absorbed on the surface of the hydrogels was removed using filter paper and the weight noted.| Swelling % = Ws − Wdry/Wdry × 100 | (1) |
2.6 Gel fraction
The weight ratio of the dried hydrogels in rinsed and unrinsed conditions can be assumed as an index of the degree of crosslinking or gel fraction. The pieces of hydrogel samples (3 × 3 cm) were dried for 6 h at 50 °C under vacuum. The same sample (3 × 3 cm) was immersed into an excess of DDW for 4 days to rinse away unreacted parts. The gels were dried again at 50 °C under vacuum. The gel fraction percentage was calculated by the following equation:20| Gel fraction % = Wf/Wi × 100 | (2) |
2.7 Blood compatibility by hemolytic activity assay
The hemolytic test was performed following the protocol of Das Purkayastha, M. et al. with a slight modification.21 Briefly, fresh goat blood from a slaughterhouse was collected in a centrifuge tube containing an anticoagulant, trisodium citrate (3.2%), and was centrifuged at 2500 rpm for 10 min. The supernatant was discarded, and only the erythrocytes were collected. The erythrocytes were further washed three times with PBS (pH 7.4). A 5% (v/v) suspension of erythrocytes in PBS was prepared; 0.95 ml of this erythrocyte solution was placed in a 1.5 ml centrifuge tube, and 0.05 ml of sample (0.5 mg dissolved in 1 ml of 0.5% DMSO) was added to it. The tubes were then incubated for 1 h at 37 °C. Triton X-100 (0.2%) and PBS were taken as the positive and negative controls, respectively, for comparison. After incubation, the tubes were subjected to centrifugation at 2500 rpm for 10 min. Then, 0.2 ml of the supernatant was added to a 96-well plate, and finally the absorbance was taken at 570 nm in a UV-visible spectrophotometer (Shimadzu UV-2550 UV-vis spectrophotometer).| Hemolysis % = [(sample O.D − negative control O.D)/(positive control O.D − negative control O.D] × 100 | (3) |
2.8 Loading of vitamin B12
The method of soaking or equilibration was employed for vitamin B12 loading. In this method, the amount of buffer necessary for complete swelling of the nanocomposite hydrogel was determined.22 Dry hydrogel was placed in the drug solution at a concentration of 0.125 wt%, prepared in the buffer solution of pH 7.0 and left until all the drug solution was sucked up. Then the completely swollen hydrogel loaded with the vitamin B12 was placed in an oven at 30 °C for drying overnight.2.9 In vitro drug release studies
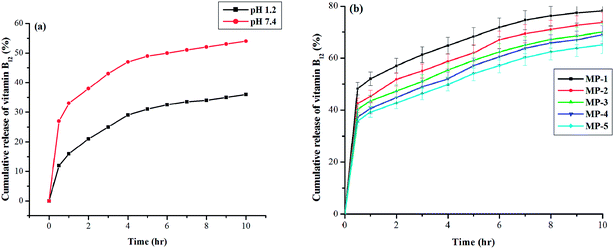
The dried vitamin B12 loaded hydrogel was immersed into 30 ml solution of different pH, namely, 1.2 and 7.4. At scheduled time intervals, 5 ml solution was withdrawn and assayed spectrophotometrically at 362 nm by using a UV-Visible spectrophotometer (UV-2001, Hitachi, Japan) for the determination of the cumulative amount of drug release. To maintain a constant volume, 5 ml of distilled water and the solution having the same pH was returned to the container. The amount of vitamin B12 released from the nanocomposite hydrogel was calculated from a calculated from a previously calibrated standard curve.233. Results and discussion
3.1 FT-IR spectra analysis
To investigate the formation of CMC-g-PAA/OMMT nanocomposite, FTIR spectra of OMMT nanoclay, CMC-g-PAA copolymer and CMC-g-PAA/OMMT nanocomposite hydrogels were acquired and are shown in Fig. 2. In the FTIR spectrum (Fig. 1a) of OMMT the broad band centered near 3400 cm−1 is due to the –OH stretching mode of the interlayer water. The overlaid absorption peak in the region of 1637 cm−1 is assigned to the –OH bending mode of adsorbed water. The characteristic peak at 1108 cm−1 is due to the Si–O–Si stretching and out of plane Si–O–Si stretching mode for montmorillonite. The band in the region of 830 cm−1 is due to the Si–O–Al stretching mode for montmorillonite. The FTIR peak at 522 cm−1 is assigned to the Si–O–Al bending vibration. | ||
| Fig. 2 FTIR spectra of (a) Pure OMMT nanoclay, (b) CMC-g-PAA copolymer and (c) CMC-g-PAA/OMMT nanocomposite hydrogels. | ||
Moreover, the FTIR spectrum of CMC-g-PAA copolymer (Fig. 2b) shows characteristic peaks at 1741 cm−1 due to the presence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and the peak observed at 613 cm−1 is due to the –OH out of plane vibration of the carboxylic groups of PAA, which confirms that the grafting reaction occurred. The characteristic peaks at 3552 cm−1 and 3431 cm−1 are due to the –OH stretching vibration of CMC and PAA, respectively, which also confirms the grafting of PAA onto CMC. Other characteristic peaks at 1657 and 1457 cm−1 are assigned to the asymmetrical and symmetrical stretching of COO− groups.
O stretching vibration and the peak observed at 613 cm−1 is due to the –OH out of plane vibration of the carboxylic groups of PAA, which confirms that the grafting reaction occurred. The characteristic peaks at 3552 cm−1 and 3431 cm−1 are due to the –OH stretching vibration of CMC and PAA, respectively, which also confirms the grafting of PAA onto CMC. Other characteristic peaks at 1657 and 1457 cm−1 are assigned to the asymmetrical and symmetrical stretching of COO− groups.
In the FTIR spectrum of nanocomposite hydrogel (Fig. 2c), it has been observed that the spectrum of the CMC-g-PAA/OMMT nanocomposite hydrogel shows variations in intensity and shifting of peaks from 3552 to 3435.8 cm−1 appears due to the –OH stretching vibration of CMC. The intensities of peaks due to Si–O and Al–O of MMT are reduced as shown in Fig. 2c. It can be explained that the –OH of MMT could react with an acrylic monomer and the MMT particles could chemically bond with the polymer chains to form the CMC/polymer/OMMT network.24 The very intense characteristic band at 1550 cm−1 is due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching in the carboxylate anion that is reconfirmed by the peak at 1450 cm−1, which is related to the symmetric stretching mode of the carboxylate anion.
O asymmetric stretching in the carboxylate anion that is reconfirmed by the peak at 1450 cm−1, which is related to the symmetric stretching mode of the carboxylate anion.
3.2 X-Ray Diffraction (XRD) analysis
XRD experiments were run to gather information on the structure of the CMC-g-PAA/OMMT nanocomposite hydrogel. Pure OMMT (Fig. 3a) displays a diffraction peak at 6.24°, corresponding to a d-spacing of 14.12 Å, but the presence of 4.0 wt% of the mineral clay (OMMT) in the polymer matrix resulted in a shift of this diffraction peak towards smaller angle 2θ = 4.69°, corresponding to a d-spacing of 18.6 Å (Fig. 3b). This increase in d-spacing indicates the increasing of layer spacing due to the intercalation or exfoliation of organophilic nanoclay into the CMC-g-PAA copolymer matrix.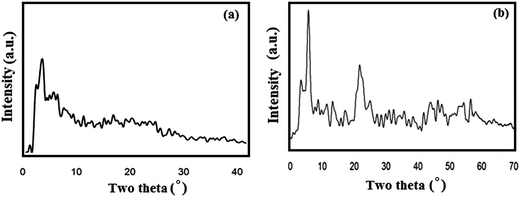 | ||
| Fig. 3 XRD patterns of (a) pure OMMT nanoclay and (b) CMC-g-PAA/OMMT nanocomposite hydrogels (10 wt% OMMT nanoclay). | ||
3.3 SEM observations
The morphologies of the copolymer hydrogel and OMMT nanoclay incorporated hydrogels were studied by SEM and the images are shown in Fig. 4a and b. These pictures indicate the change in the surface morphology of the prepared hydrogels after the incorporation of nanoclay. A rough surface morphology is observed and some pores and gaps can be observed in the micrograph of OMMT nanoclay incorporated hydrogel. This observation implies that incorporating OMMT nanoclay is favourable for improving the surface structure of the hydrogel. | ||
| Fig. 4 SEM images of (a) CMC-g-PAA copolymer and (b) CMC-g-PAA/OMMT nanocomposite hydrogels (10 wt% OMMT nanoclay). | ||
3.4 Mechanical properties
Dynamic mechanical analysis was performed on the nanocomposite hydrogel to provide quantitative information on the viscoelastic and rheologic properties of the hydrogel by measuring the mechanical response of the samples as they were deformed. Fig. 5a and b depict the isothermal DMA-response of the nanocomposite hydrogels as a function of frequency in terms of storage modulus (G′) and loss modulus (G′′). The elastic component (G′) is related to the stiffness of the material, while G′′ (the viscous component) is associated with the dissipation of energy, as heat, due to internal friction at the molecular level. | ||
| Fig. 5 Dynamic mechanical analysis of CMC-g-PAA/OMMT nanocomposite hydrogels as a function of frequency: (a) storage modulus (G′) and (b) loss modulus (G′′) with OMMT nanoclay content from 0–20 wt%. | ||
It was observed, for all the compositions, that the storage (G′) tends to increase with increasing frequency. It was also observed that the elastic modulus increased significantly with increased amount of nanoclay content, indicating that the nanoclay (OMMT) acts as an additional cross-linking agent and improves the stiffness of the nanocomposite hydrogels. Lima et al. developed stimuli-responsive chitosan–starch injectable hydrogels combined with encapsulated adipose-derived stromal cells with better mechanical properties. They found that for all the compositions, the storage modulus (G′) tended to increase with increasing frequency.25
The loss (viscous) modulus was also dependent on the cross-linking density and in the whole range of deformation, the loss moduli also increased with increasing nanoclay content. But this was lower than the storage modulus as the elastic properties dominate. The maximum G′ of the nanocomposite hydrogel reached 18 020 Pa at γ = 1%, ω = 10 Hz and 20 wt% OMMT nanoclay and the maximum value of G′′ was 9566 Pa at γ = 1%, ω = 10 Hz and 20 wt% OMMT nanoclay content, which was remarkably lower than the value for G′. However, larger values of G′ and G′′ were observed for the clay incorporated hydrogel, indicating the improved mechanical properties. The values indicate the formation of a strong nanocomposite hydrogel.
3.5 Swelling properties of the hydrogels
3.6 Determination of gel fraction
A typical dependency of gel fraction on the quantity of clay incorporated into hydrogels is given in Table 3. It was observed that the gel fraction of the prepared nanocomposite hydrogels gradually increased with increasing clay content. The gel fraction data revealed that the presence of clay within the three dimensional network of the hydrogel caused an increase in crosslinking density and created a more entangled structure. As the organo-modified MMT nanoclay was added to the CMC-g-polyacrylic acid hydrogel, strong interactions were developed between the functional groups of the nanoclay and the polymer matrix, which may have caused an increase in the gel fraction.263.7 Blood compatibility studies
Continuous efforts have been made to design novel biomaterials with superior blood compatibility by various research groups. Biomedical applications, such as drug delivery and tissue engineering, are the well-known examples of the use of hydrogels, which have been extensively reported.27 Since these applications involve the use of humans or other animals, it is important to study their biocompatibility with blood. The in vitro blood compatibility studies were carried out for the prepared nanocomposite hydrogels by using hemolysis tests as described in Section 2.7.The hemolysis test was performed both for the native hydrogel and OMMT nanoclay incorporated nanocomposite hydrogel with different concentrations. Significantly less hemolysis activity was found for the clay incorporated hydrogel (10 wt%). For all samples, contact with blood showed a mean hemolysis value less than 0.5%. The test showed very low hemolysis activity and the data obtained are at the permissible limit as shown in Fig. 7a. Images showing precipitated RBCs at the end of the hemolysis experiment are also given in Fig. 7b.
3.8 In vitro release of vitamin B12 from CMC-g-PAA/OMMT nanocomposite hydrogel
3.9 Determination of release kinetics of vitamin B12
In order to study the vitamin B12 transport mechanism from the nanocomposite hydrogels with different crosslinker content, the data was modeled by the Ritger–Peppas equation:30| Mt/Mα = ktn | (4) |
4. Conclusions
In this study, nanocomposite hydrogels of CMC, partially neutralized acrylic acid and layered organically modified OMMT clay were prepared by free radical graft polymerization. X-ray diffraction analysis showed that the nanocomposite forms a partially exfoliated or intercalated structure. Moreover, SEM studies demonstrated an improvement of surface properties of the nanocomposite as compared to the composite. The evaluation of the dynamic mechanical analysis of the nanocomposite hydrogels showed an increase in the storage modulus within increasing frequency and OMMT nanoclay content, clearly demonstrating good mechanical behavior. Swelling percentage of the nanocomposite hydrogel initially increased with an increase in initiator (KPS) content, but decreased after a definite amount of initiator. Furthermore, the swelling percentage of the nanocomposite hydrogel was found to decrease with increasing crosslinker (MBA) and nanoclay (OMMT) content. The gel fraction of the nanocomposite hydrogel was determined and was found to increase with increasing nanoclay content from 0 to 20 wt%. Blood compatibility of the prepared hydrogels was improved after the incorporation of nanoclay as confirmed by in vitro experiments of percentage hemolysis. The vitamin B12 release behavior exhibited a considerable dependence on pH of the medium and cross-linking density of the nanocomposite hydrogels. The release behavior was found to be greater in basic medium (pH 7.4) than in the acidic medium (pH 1.2). Kinetic study of release behavior showed an increase in the ‘n’ value from 0.63 to 0.87 exhibiting a non-Fickian transport mechanism with an increase in cross-linker content. Therefore, the results obtained in this work lead us to the conclusion that CMC-g-PAA/OMMT nanocomposite hydrogels can be a promising platform for the development of pH-responsive drug delivery systems.Acknowledgements
One of the authors (M. Boruah), is grateful to the CSIR, India (Sanction no. 09/796/(0045)/2013/EMR-I), for providing Senior Research Fellowship as a financial support to carry out this study.References
- N. Ravi, A. Mitra, P. Hamilton and F. Horkay, J. Polym. Sci., Part B: Polym. Phys., 2002, 23, 2677–2684 CrossRef PubMed
.
- H. M. Kang, Y. L. Cai and P. S. Liu, Carbohydr. Res., 2006, 341, 2851–2857 CrossRef CAS PubMed
.
- Y. W. Lin, Q. Chen and H. B. Luo, Carbohydr. Res., 2007, 342, 87–95 CrossRef CAS PubMed
.
- W. S. Cai and R. B. Gupta, J. Appl. Polym. Sci., 2002, 83, 169–178 CrossRef CAS PubMed
.
- S. J. Kim, S. J. Park, I. Y. Kim, M. S. Shin and S. I. Kim, J. Appl. Polym. Sci., 2002, 83, 169–178 CrossRef PubMed
.
- K. Engin, D. B. Leeper, J. R. Cater, A. J. Thistlethwaite, L. Tupchong and J. D. McFarlane, Int. J. Hyperthermia, 1995, 11, 211–216 CrossRef CAS PubMed
.
- M. D. Kurkuri and T. M. Aminabhavi, J. Appl. Polym. Sci., 2004, 91, 4091–4097 CrossRef CAS PubMed
.
- M. M. Amiji, Biomaterials, 1995, 16, 593–599 CrossRef CAS
.
- J. H. Changa, Y. U. Ana and D. Cho, Polymer, 2003, 44, 3715–3719 CrossRef
.
- Y. Mansoori, S. V. Atghia, M. R. Zamanloo, Gh. Imanzadeh and M. Sirousazar, Eur. Polym. J., 2010, 46, 1844–1853 CrossRef CAS PubMed
.
- B. K. G. Theng, Formation and properties of clay–polymer complexes, Elsevier, Amsterdam, 2nd edn, 1979, vol. 4, pp. 1–8 Search PubMed
.
- N. Ogata, S. Kawakage and T. Ogihara, J. Appl. Polym. Sci., 1997, 66, 573–581 CrossRef CAS
.
- Z. Zhang, L. Liao and Z. Xia, Appl. Clay Sci., 2010, 50, 576–581 CrossRef CAS PubMed
.
- M. Miki, H. Tamai, M. Mino, Y. Yamamoto and E. Niki, Arch. Biochem. Biophys., 1987, 258, 373–377 CrossRef CAS
.
- G. Crini, Prog. Polym. Sci., 2005, 30, 38–70 CrossRef CAS PubMed
.
- M. Sadeghi and M. Yarahmadi, Afr. J. Biotechnol., 2011, 10, 12085–12093 CAS
.
- M. Wang and L. Wang, Water Sci. Eng., 2013, 6, 272–282 CAS
.
- M. Irani, H. Ismail and Z. Ahmad, Polym. Test., 2013, 32, 502–512 CrossRef CAS PubMed
.
- A. Samakande, P. C. Hartmann, V. Cloete and R. D. Sanderson, Polymer, 2007, 48, 1490–1494 CrossRef CAS PubMed
.
- M. Kokabi, M. Sirousazar and Z. M. Hassan, Eur. Polym. J., 2007, 43, 773–781 CrossRef CAS PubMed
.
- M. D. Purkayastha, S. Das, A. K. Manhar, D. Deka, M. Mandal and C. L. Mahanta, J. Agric. Food Chem., 2013, 61, 10746–10756 CrossRef PubMed
.
- S. K. Bajpai, M. Bajpai and L. Sharma, Iran. Polym. J., 2007, 16, 521–527 CAS
.
- R. van Dijkhuizen-Radersma, F. L. A. M. A. Peters, N. A. Stienstra, D. W. Grijpma, J. Feijen, K. de Groot and J. M. Bezemer, Biomaterials, 2002, 23, 1527–1536 CrossRef CAS
.
- M. Boruah, M. Mili, S. Sharma, B. Gogoi and S. K. Dolui, Polym. Compos., 2014 DOI:10.1002/pc.22909
.
- H. Lima, S. G. Caridade, J. F. Manoab and R. L. Reis, Soft Matter, 2010, 6, 5184–5195 RSC
.
- M. Kurecic and M. S. Smole, Polymer Nanocomposite Hydrogels for Water Purification, Nanocomposites – New Trends and Developments, ed. F. Ebrahimi, 1st edn, 2012, vol. 1, ch. 7, pp. 161–185 Search PubMed
.
- C. H. Lin, W. C. Jao, Y. H. Yeh, W. C. Lin and M. C. Yang, Colloids Surf., B, 2009, 70, 87–95 CrossRef PubMed
.
- L. Wang, J. Zhang and A. Wang, Colloids Surf., A, 2008, 322, 47–51 CrossRef CAS PubMed
.
- R. Wang, Q. Fu, X. Chen, Y. Gao and K. Dong, RSC Adv., 2012, 2, 7772–7780 RSC
.
- P. L. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 23–36 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2014 |