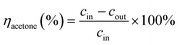Catalytic oxidation of acetone over CuCeOx nanofibers prepared by an electrospinning method
Rui Qin,
Jinghuan Chen,
Xiang Gao*,
Xinbo Zhu,
Xinning Yu and
Kefa Cen
State Key Laboratory of Clean Energy Utilization of Zhejiang University, Hangzhou, 310027, P. R. China. E-mail: xgao1@zju.edu.cn
First published on 12th August 2014
Abstract
A series of CuCeOx nanofiber catalysts with different Cu/(Cu + Ce) molar ratios were synthesized by an electrospinning method. The catalysts were evaluated for acetone oxidation at different temperatures (100–280 °C) with a GHSV of 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml g−1 h−1. The results showed that nanofiber catalysts possessed better catalytic performance than catalysts prepared by urea-nitrate combustion and sol–gel methods. An appropriate Cu/(Cu + Ce) molar ratio could greatly improve the activity of the nanofiber catalysts, and a significant improvement of the activity was obtained on the Cu0.50Ce0.50Ox nanofiber catalyst (∼100% acetone conversion at 270 °C). Characteristic analysis of prepared catalysts suggested (1) a special nanofibrous morphology with large specific surface area; (2) abundant oxygen vacancies and (3) cerium ions with unusual oxidation states were the main factors that would affect catalytic activity of CuCeOx nanofibers catalysts.
000 ml g−1 h−1. The results showed that nanofiber catalysts possessed better catalytic performance than catalysts prepared by urea-nitrate combustion and sol–gel methods. An appropriate Cu/(Cu + Ce) molar ratio could greatly improve the activity of the nanofiber catalysts, and a significant improvement of the activity was obtained on the Cu0.50Ce0.50Ox nanofiber catalyst (∼100% acetone conversion at 270 °C). Characteristic analysis of prepared catalysts suggested (1) a special nanofibrous morphology with large specific surface area; (2) abundant oxygen vacancies and (3) cerium ions with unusual oxidation states were the main factors that would affect catalytic activity of CuCeOx nanofibers catalysts.
1 Introduction
Volatile organic compounds (VOCs) are massively emitted via human activities like industry production, fuel combustion and oil storage. VOCs are precursors of photochemical smog, which threaten the environment and human health seriously. Exposure to VOCs may cause carcinogenic, mutagenic, and teratogenic effects on human body due to their toxicity.1 Researchers have explored various methods for VOCs elimination,2–4 among these strategies, catalytic oxidation is one of the most effective and economic ways for VOCs abatement in large volumes of air.5 In this study, acetone was selected as the model VOC since it is one of the most abundant VOC in the atmosphere.6Noble metals and transition metal oxides have been studied for VOCs catalytic oxidation extensively in recent decades.7 Noble metals have been proven to be more active at lower temperatures than transition metal oxides, but their high cost and low resistance to halogens limit their application.8,9 Otherwise, some transition metal oxide catalysts have also been found to be active for VOCs catalytic oxidation.10,11 Among them, copper oxide and cerium oxide catalysts were researched intensively. Since copper oxide catalysts possess high activity for VOCs catalytic oxidation12 and cerium oxide catalysts have high oxygen storage capacity and facile reducibility of Ce4+/Ce3+ compared to other fluorite-type oxides.13 In addition, researchers even found that Cu–Ce binary metal oxide catalysts possess high activity, which is even comparable to supported noble metal catalysts for oxidation reactions, as the shift between Ce4+ and Ce3+ becomes easier when transition metal ions are added in ceria lattice.14–16
Various methods have been used in preparation of metal oxide catalysts, including sol–gel and combustion methods. However, these catalysts have relatively low specific surface area and their microstructure inhibits the diffusion, adsorption and desorption process of molecules to some extent. As is well known, nanofiber catalysts are widely used in photocatalytic reactions since they possess large specific surface area and one-dimensional nanofibrous morphology, which can promote the mass transfer process during reactions.17,18 Therefore, in order to improve catalytic performance, it is necessary to find an appropriate method to synthesize metal oxide nanofiber catalysts. Among a variety of techniques, the electrospinning technique has been proven as an effective way to produce fibrous metal oxide in nanoscale.19,20 This processing involved the following steps: (1) preparation of sol solution with a suitable inorganic precursor and polymer content, and achieving the right rheology for electrospinning; (2) spinning of the solution to obtain polymer/inorganic fibers; and (3) calcinations of the composite fibers to obtain the final oxide nanofibers.20
In this work, one-dimensional CuCeOx nanoscale fibers with different Cu/(Cu + Ce) ratios were successfully prepared by an electrospinning method. The morphological, structural and chemical characteristics of the catalysts were investigated by SEM, EDX, TEM, XRD, XPS, N2 sorption, H2-TPR, and the catalytic performance for acetone oxidation was examined in a fixed-bed reactor. The results showed that the electrospinning method endowed CuCeOx nanofibers catalysts with specific one-dimensional nanofibrous morphology. Then, an attempt to establish the relationship between structure and catalytic performance for acetone oxidation was made. In addition, this study may be helpful for designing or synthesizing novel metal oxide catalysts and expanding the application of metal oxide catalysts in filtration and separation.21
2 Experimental
2.1 Catalyst preparation
CuCeOx nanofibers catalysts were synthesized via an electrospinning method. Firstly, appropriate amounts of Ce(NO3)3·6H2O and Cu(NO3)2·3H2O were dissolved in 6 ml anhydrous alcohol and 10 ml deionized water simultaneously. Total nitrate salts were 0.01 mol and the molar ratios of Cu/(Cu + Ce) were 0, 0.15, 0.30, 0.50, 0.70 and 0.85. (It should be mentioned that for the same preparation process, the viscosity of a sol solution of pure Cu(NO3)2·3H2O is too low to adapt to our electrospinning process; thus, the preparation of pure CuO nanofibers is not available.) After magnetically stirring for 1 h, 2 g of polyvinylpyrrolidone (PVP, Mw = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was added in the solution. Then, the mixtures were agitatedly stirred for 12 h at room temperature to obtain a homogenous and translucent solution. CuCe/PVP as-spun nanofibers were prepared by electrospinning the viscous sol–gel solution between a syringe and an aluminum foil attached on a roller (rotating speed 100 rpm). The distance between the tip of spinneret (21G) and the aluminum foil was 150 mm. A DC high-voltage supply connected on the needle generated a negative voltage of 15 kV and the flow rate was controlled by a syringe pump at a constant flow of 0.5 ml h−1. This electrospinning process was carried out in ambient air at room temperature. Then, the as-spun fibers were dried at 20 °C for 24 h and calcined in air at 500 °C for 4 h to obtain CuCeOx nanofibers (denoted as NFs).
000) was added in the solution. Then, the mixtures were agitatedly stirred for 12 h at room temperature to obtain a homogenous and translucent solution. CuCe/PVP as-spun nanofibers were prepared by electrospinning the viscous sol–gel solution between a syringe and an aluminum foil attached on a roller (rotating speed 100 rpm). The distance between the tip of spinneret (21G) and the aluminum foil was 150 mm. A DC high-voltage supply connected on the needle generated a negative voltage of 15 kV and the flow rate was controlled by a syringe pump at a constant flow of 0.5 ml h−1. This electrospinning process was carried out in ambient air at room temperature. Then, the as-spun fibers were dried at 20 °C for 24 h and calcined in air at 500 °C for 4 h to obtain CuCeOx nanofibers (denoted as NFs).
For comparison, the CuCeOx catalysts with Cu/(Cu + Ce) molar ratio of 0.5 were also prepared by urea-nitrate combustion (denoted as cmb) and sol–gel (denoted as sol) methods.13,22
2.2 Catalyst characterizations
Field emission scanning electron microscopy (FE-SEM) images and energy dispersive X-ray spectroscopy (EDX) data were recorded on a SIRION microscope with a voltage of 25 kV coupled to an X-ray detector.Transmission electron microscopy (TEM) was performed using a Hitachi H-9500 microscope operating with an acceleration voltage of 300 kV. For the TEM measurement, the samples were prepared by ultrasonication in ethanol, followed by evaporating a drop of the resultant suspension onto a carbon-coated copper grid.
BET surface areas were measured by N2 adsorption and desorption at liquid nitrogen temperature (77 K) with Autosorb-1-C (Quantachrome Instrument Crop.). All samples were degassed under vacuum at 300 °C for 2 h before the measurement.
X-ray diffraction (XRD) patterns were recorded on a PANalytical X'Pert PRO XRD system using Cu Kα radiation (λ = 0.15418 nm) in the 2θ range of 10–80° (scanning rate of 4° min−1).
X-ray photoelectron spectroscopy (XPS) data were obtained with a Thermo ESCALAB 250Xi using Al Kα as the excitation radiation at a constant pass energy of 1486.6 eV. The binding energies were calculated with reference to the energy of the C 1s peak of contaminant carbon at 284.6 eV.
Temperature programmed reduction of hydrogen (H2-TPR) experiments were performed in a quartz reactor in 10% H2/Ar with a flow rate of 30 ml min−1, and 15 mg of catalyst was used. Prior to reduction, catalyst was heated in a 10% O2/Ar flow (30 ml min−1) from room temperature to 300 °C and held for 30 min, and then treated in a Ar stream at room temperature for 30 min to remove any residual oxygen. The samples were heated from 50 °C to 850 °C at a flow rate of 10 °C min−1. The amount of H2 consumption was measured using a thermal conductivity detector (TCD).
2.3 Catalytic activity
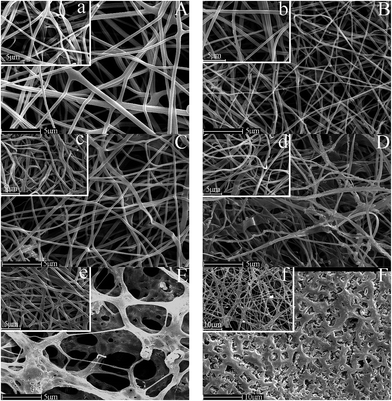
All catalytic performance tests were performed in a fixed bed tubular quartz reactor (6 mm i.d.) at atmospheric pressure. 35 mg of the catalyst (40–60 mesh) was placed in the middle of the reactor and the total flow rate was kept at 46 ml min−1, corresponding to a gas hourly space velocity (GHSV) that was 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml g−1 h−1. The feed gas mixture contained 500 ppm acetone, 5% O2, and the balance was N2. The temperatures of the catalyst bed and tubular electric furnace were monitored automatically by E-type thermocouples. The inlet and outlet concentrations of acetone were analyzed on-line by a gas chromatograph (Agilent 7890A, USA) equipped with a flame ionization detector (FID) and a capillary column of HP-Innowax (Agilent, USA) operated at 60 °C. The acetone removal efficiency (ηacetone) is calculated as follows:
000 ml g−1 h−1. The feed gas mixture contained 500 ppm acetone, 5% O2, and the balance was N2. The temperatures of the catalyst bed and tubular electric furnace were monitored automatically by E-type thermocouples. The inlet and outlet concentrations of acetone were analyzed on-line by a gas chromatograph (Agilent 7890A, USA) equipped with a flame ionization detector (FID) and a capillary column of HP-Innowax (Agilent, USA) operated at 60 °C. The acetone removal efficiency (ηacetone) is calculated as follows:cin, cout—inlet and outlet concentration of acetone.
Blank test was conducted with crushed quartz sand (40–60 mesh) to guarantee the credibility of this test. The results showed that there was no thermal destruction of acetone in the same feed gas below 280 °C. Before each test, catalysts were first placed in the fixed-bed tube with the feed stream passing and stabilized for 1 h. After stabilization, the temperature was increased at a heating rate of 5 °C min−1, and all the data obtained at each temperature were the average of three measurements which were stabilized for 20 min respectively.
3 Results and discussion
3.1 Morphology characterization
The morphology and microstructure of the as-spun (Fig. 1a–f) and calcinated nanofibers (Fig. 1A–F) were investigated by SEM. The micrographs demonstrated that the length of nanofibers is of the order of several millimeters, and their average diameter (denoted as D) is of the order of about hundreds of nanometers (Table 1). The pure CeO2 nanofibers possess one-dimensional nanofibrous morphology and the average diameter of as-spun CeO2 nanofibers is 610 nm. When copper nitrates were added, the average diameter of as-spun nanofibers decreases. With the increase of Cu/(Cu + Ce) molar ratio from 0.15 to 0.70, the average diameter decreases from 590 to 525 nm; however, when the copper content further increased, several coarse grains are formed on the nanofibers and a part of the fibers tangle with each other to form arachnoid morphology. After calcination, the diameter of nanofibers reduces about 200 nm, probably caused by the decomposition of PVP and formation of copper and cerium oxide crystals during programmed temperature process. With the increase of Cu/(Cu + Ce) molar ratio from 0 to 0.50, the average diameter of calcinated nanofibers decreases from 460 to 295 nm. With further increase of Cu/(Cu + Ce) molar ratio up to 0.70, their fibrous morphology was deformed to arachnoid morphology with coarse grains. When the Cu/(Cu + Ce) molar ratio was increased up to 0.85, nanofibers lose their nanofibrous morphology completely and form a sheet with coarse surface. The same phenomenon was also found in electrospinning of CuO/TiO2 nanofibers. Researchers have found that addition of copper salts in sol–gel solution had a negative impact on the nanofibrous morphology.23| Sample | SBET (m2 g−1) | Das-spuna (nm) | Dcalcinatedb (nm) | dporec (nm) | d111d (Å) | CuEDX (at%) | CuXPS (at%) | Oadse (%) | Ce3+f (%) | T50 (°C) | T90 (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Average diameter of as-spun nanofibers.b Average diameter of calcinated nanofibers.c Average pore diameter of nanofibers.d The lattice parameters of CeO2 (111).e The percentage of adsorbed oxygen measured by XPS.f The percentage of Ce3+ measured by XPS. | |||||||||||
| CeO2-NFs | 38.9 | 610 | 460 | 9.1 | 5.424 | — | — | 35.43 | — | 230 | 270 |
| Cu0.15-NFs | 46.3 | 590 | 345 | 8.9 | 5.420 | 19.77 | 14.42 | 42.81 | 14.10 | 205 | 240 |
| Cu0.30-NFs | 52.5 | 545 | 340 | 8.1 | 5.411 | 34.32 | 27.31 | 44.70 | 18.38 | 195 | 230 |
| Cu0.50-NFs | 74.5 | 530 | 295 | 8.4 | 5.407 | 47.99 | 33.07 | 52.43 | 18.38 | 190 | 225 |
| Cu0.70-NFs | 37.5 | 525 | — | 10.7 | 5.409 | 70.17 | 51.28 | 37.78 | 12.19 | 210 | 250 |
| Cu0.85-NFs | 24.9 | — | — | 26.1 | 5.422 | 84.27 | 77.17 | 39.75 | 5.61 | 215 | 260 |
| Cu0.50-sol | 20.2 | — | — | — | 5.420 | 49.62 | 77.67 | 33.70 | 8.00 | 220 | 260 |
| Cu0.50-cmb | 2.4 | — | — | — | 5.413 | 50.33 | 87.10 | 27.77 | 4.40 | 240 | — |
The micro-morphology of nanofibers was further investigated by TEM. As an example, the TEM images of Cu0.50-NFs are shown in Fig. 2. Fig. 2A shows that the diameter of the single nanofiber is about 120 nm and the length is >1 μm. Moreover, the inner-planar spacing was measured and shown in high resolution TEM (HRTEM) image (Fig. 2B). The lattice fringes are 0.31 nm, 0.27 nm and 0.23 nm, which correspond well with fringe spacing of CeO2 (111), (200) (JCPDS 34-0394) and CuO (111) (JCPDS 45-0937), respectively, indicating the presence of CeO2, CuO nanocrystals in the nanofibers.
3.2 XRD characterization
Fig. 3 showed the wide-angle XRD patterns of synthesized samples. The reflections at 2θ = 28.5°, 33.1°, 47.5°, 56.3°, 59.1°, 69.4°, 76.7° were detected in pure CeO2-NFs. All these peaks fitted well with CeO2 (111), (200), (220), (311), (222), (400), (331) (JCPDS 34-0394), which indicate the typical cubic fluorite-like structure of CeO2. When the Cu/(Cu + Ce) molar ratio varied from 0 to 0.50, several diffraction peaks of CeO2 were detected while the diffraction peaks of CuO were quite weak. This may be due to good dispersion of small size CuO particles on the surface of CeO2, or the incorporation of Cu ion in the CeO2 lattice in the form of Cu–Ce–O solid solution. However, when the Cu/(Cu + Ce) molar ratio was over 0.5, the CuO corresponding diffraction peaks at 2θ = 32.5°, 38.5° (JCPDS 45-0937) become obvious, indicating that copper oxides start to separate and form part of bulk CuO particles. Moreover, the lattice parameters of cerium oxide (d111) of all samples were calculated and listed in Table 1. After partial incorporation of copper ions in CeO2 lattice, d111 decreases slightly since the radius of six-coordinated Cu2+ (0.73 Å) and Cu+ (0.77 Å) is smaller than that of six-coordinated Ce3+ (1.10 Å) or Ce4+ (1.01 Å). Furthermore, the shift of diffraction peaks of CeO2 (111) toward higher degree was observed, which corresponds well with Bragg's law (2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = nλ). These evidences proved that a part of Cu ions are incorporated in CeO2 lattice and form Cu–Ce–O solid solution.24
θ = nλ). These evidences proved that a part of Cu ions are incorporated in CeO2 lattice and form Cu–Ce–O solid solution.24
 | ||
| Fig. 3 XRD patterns of (a) CeO2-NFs, (b) Cu0.15-NFs, (c) Cu0.30-NFs, (d) Cu0.50-NFs, (e) Cu0.70-NFs, (f) Cu0.85-NFs, (g) Cu0.50-sol, (h) Cu0.50-cmb. | ||
Comparing with Cu0.50-sol and Cu0.50-cmb, the diffraction peaks of nanofibers catalysts are weaker and broader, which indicates that copper and cerium oxide particles of nanofibers are smaller and better dispersed. In addition, the CeO2 lattice parameter of Cu0.50-NFs (5.407 Å), which is smaller than that of Cu0.50-sol (5.4201 Å) and Cu0.50-cmb (5.413 Å), indicates that the amount of Cu–Ce–O solid solution in Cu0.50-NFs is more than that in Cu0.50-sol and Cu0.50-cmb catalysts.
The better dispersion of copper oxide and formation of Cu–Ce–O solid solution can strengthen the interactions between CuO and CeO2 intensively, which make Ce–O and Cu–O bonds to become weaker and easier to crack and produce active oxygen species.25 These active oxygen species can improve the reducibility and catalytic activity of CuCeOx catalysts.
3.3 N2 sorption and EDX characterization
The adsorption–desorption isotherms of nanofiber catalysts were illustrated in Fig. 4. All nanofiber catalysts showed typical type IV isotherms. Their hysteresis could be identified as H3 type on the basis of IUPAC classification, which indicates the possible formation of open slit-shaped pores. The average pore diameter (dpore) and specific surface areas (SBET) of nanofiber catalysts were shown in Table 1 based on the calculation from the isotherms. The average pore diameter of nanofiber catalysts varies from 8.1 to 26.1 nm with different copper content. The specific surface area of nanofiber catalysts is around 24.9–74.5 m2 g−1. It increases gradually from 38.9 to 74.5 m2 g−1 with increase of the Cu/(Cu + Ce) molar ratio from 0 to 0.50 with decrease in the average diameter of nanofibers. However, when the Cu/(Cu + Ce) molar ratio further increased from 0.50 to 0.85, SBET decreases drastically. This may be due to the destruction of nanofibrous morphology caused by introducing of excess copper ions. Moreover, the specific surface area of nanofibers is much higher than that of Cu0.50-sol (20.2 m2 g−1) and Cu0.50-cmb (2.4 m2 g−1). Therefore, it can be deduced that the electrospinning method endows the Cu–Ce composite catalyst with specific nanofibrous morphology and enough surface area, which in turn promote the activity of reactions. | ||
| Fig. 4 N2 adsorption–desorption isotherms of (a) CeO2-NFs, (b) Cu0.15-NFs, (c) Cu0.30-NFs, (d) Cu0.50-NFs, (e) Cu0.70-NFs, (f) Cu0.85-NFs. | ||
The bulk composition of the CuCeOx catalysts was determined by EDX analysis. Table 1 summarized the Cu atomic relative ratio (CuEDX) determined by EDX. The results showed that the nominal compositions of all catalysts agree well with the relative molar ratio determined by EDX except for Cu0.15-NFs and Cu0.30-NFs.
3.4 XPS characterization
The information of surface composition and chemical state of the CuCeOx catalysts were studied by XPS. The measured elements in the samples are oxygen (O 1s), cerium (Ce 3d), copper (Cu 2p), and carbon (C 1s) in the binding energy from 0 to 1000 eV (other than CeO2-NFs). Oxygen, cerium, copper were expected from the chemical composition of the synthesized CuCeOx catalysts. The C 1s peak was used for calibration.The O 1s spectras of various samples, which can be resolved into two peaks by deconvolution, were shown in Fig. 5A. The peaks at 529.4 eV can be attributed to lattice oxygen (denoted as Olatt), and the shoulder peaks at 531.2–532.1 eV can be assigned to the active surface adsorbed oxygen (denoted as Oads).26 The active Oads contents (Oads/(Olatt + Oads)) at the surface of synthesized catalysts were listed in Table 1. With the increase of the Cu/(Cu + Ce) molar ratio from 0 to 0.50, the active Oads percentage of nanofibers increases from 35.34% to 52.43%, and then it decreases with further increase in the copper content. Among all the nanofibers, Cu0.50-NFs possesses the largest amount of Oads (52.43%), which is more than that of Cu0.50-sol and Cu0.50-cmb.
 | ||
| Fig. 5 (A) O 1s, (B) Ce 3d, (C) Cu 2p XPS spectra of (a) CeO2-NFs, (b) Cu0.15-NFs, (c) Cu0.30-NFs, (d) Cu0.50-NFs, (e) Cu0.70-NFs, (f) Cu0.85-NFs, (g) Cu0.50-sol, (h) Cu0.50-cmb. | ||
According to the literature,27 the complex spectrum of Ce 3d could be decomposed into ten peaks. The two sets of spin–orbital multiplets, corresponding to the 3d3/2 and 3d5/2 contributions are labeled as u and v, respectively. The u2, u0, v2, v0 peaks can be ascribed to Ce3+ and u1, u3, u4, v1, v3, v4 peaks can be attributed to Ce4+. The Ce3+ relative content was then estimated by Ce3+/(Ce3++ Ce4+) × 100%, where Ce3+ = (u2 + u0 + v2 + v0) and Ce4+ = (u1 + u3 + u4 + v1 + v3 + v4). Ce3+ contents of CuCeOx catalysts at the surface were listed in Table 1, the results illustrated that Cu0.30-NFs and Cu0.50-NFs possess the largest amount of Ce3+ (18.38%) among all the nanofibers, which is much higher than that of Cu0.50-sol (7.96%) and Cu0.50-cmb (4.40%). The existence of Ce3+ in CeO2 indicates the formation of oxygen vacancies.28 These oxygen vacancies, which are directly related to the catalytic activity at low temperatures,29 may induce more surface adsorbed oxygen species.30
The Cu 2p binding energy of various catalysts was shown in Fig. 5C. The principal peaks at 934.0 eV, 934.6 eV can be identified as Cu2+ 2p3/2, whereas the peaks at 953.7 eV stand for Cu2+ 2p1/2 and the shake-up peaks from 940.0 eV to 945.0 eV indicate the presence of reduced copper species.
The relative atomic ratios of Cu at the surface are summarized in Table 1. The results showed CuXPS ratios of nanofiber catalysts are lower than their corresponding nominal ratios and CuEDX ratios, suggesting that Cu–Ce–O solid solution could be formed on the surface of nanofiber catalysts, which was also discovered by other researchers.24 However, the CuXPS ratios of catalysts prepared by sol–gel and the urea-nitrate combustion method are much higher than their corresponding nominal ratio and CuEDX ratios, indicating the enrichment of Cu species or formation of large bulk of copper oxide on the surface of these catalysts.
Comparing with the XPS results of O 1s and Ce 3d, it can be concluded that the active Oads content is enhanced with the increase of Ce3+ relative content, and the augmentation of these two species can be explained by charge compensation theory. In the case of CuCeOx catalysts, active copper species can be incorporated in the cerium oxide lattice and destroy the lattice structure, and thus influence the electroneutrality. In order to retain electroneutrality, charge compensation achieved by either formation of oxygen vacancies or shift of cerium to a lower oxidation state is required. Consequently, the active oxygen adsorbed on surface oxygen vacancies and cerium ions with unusual oxidation state (Ce3+) can increase simultaneously.
3.5 TPR characterization
In order to check the reduction properties of the prepared catalysts, all samples were investigated by H2-TPR. The H2-TPR profiles were displayed in Fig. 6. H2-TPR patterns illustrated pure cerium oxide nanofiber catalysts possess three TPR peaks above 300 °C. The peaks at 400 and 501 °C are attributed to the reduction of surface oxygen species, and the peak at 743 °C is ascribed to the reduction of bulk oxygen species.31 After the addition of copper, the reduction peaks ascribed to cerium oxide could also be seen in the H2-TPR profiles. However, two or three new reduction peaks emerged in the nanofiber catalysts below 300 °C. The hydrogen consumption peaks (with shoulder peaks) at the range of 190–285 °C can be attributed to the reduction of bulk CuO. The reduction peaks of all CuCeOx nanofibers catalysts during the range of 145–183 °C indicate the highly dispersed active Cu species.32 Interestingly, with the increase of copper content, the main peaks shift to higher temperatures and the minor peaks, which at lower temperatures than main peaks, disappear gradually . This behavior fits well with what has been found in CuCeOx catalysts prepared by the combustion method.14 | ||
| Fig. 6 H2-TPR patterns of (a) CeO2-NFs, (b) Cu0.15-NFs, (c) Cu0.30-NFs, (d) Cu0.50-NFs, (e) Cu0.70-NFs, (f) Cu0.85-NFs, (g) Cu0.50-sol, (h) Cu0.50-cmb. | ||
The reduction features of Cu0.50-sol and Cu0.50-cmb are different from nanofibers, they have only one reduction peak below 300 °C, at 235 °C and 276 °C, respectively, suggesting a large amount of bulk CuO was formed in these two catalysts. In addition, the main reduction peaks of Cu0.50-sol and Cu0.50-cmb appear at higher temperatures than the major peak (at 203 °C) of Cu0.50-NFs, indicating the reducibility of nanofiber catalysts is much stronger than catalyst synthesized via sol–gel and the urea-nitrate combustion method.
3.6 Catalytic performance
The catalytic activities of prepared catalysts were tested in the temperature range from 100 to 280 °C. The acetone conversion pattern is shown in Fig. 7, and the T50 and T90 (temperatures at 50% and 90% conversion of acetone) of catalyst calculated from the conversion curves are listed in Table 1. In the tested temperature range, CeO2-NFs possess the lowest catalytic activity among all the nanofibers (T90 = 270 °C), and with increase in Cu/(Cu + Ce) molar ratio from 0 to 0.50, their acetone conversion curves shift progressively toward lower temperatures. Furthermore, increasing copper content diminishes the catalytic activity of nanofibers. Cu0.50-NFs possesses the best acetone oxidation activity (T90 = 225 °C), and 100% acetone conversion is achieved at around 270 °C. The acetone conversion activity sequence over the nanofibers catalysts is Cu0.50-NFs > Cu0.30-NFs > Cu0.15-NFs > Cu0.70-NFs > Cu0.85-NFs > CeO2-NFs. In addition, T50 acetone conversion of Cu0.50-sol and Cu0.50-cmb are 220 °C and 240 °C, respectively, which are much lower than that of Cu0.50-NFs (T50 = 190 °C). Since the GHSV and concentrations of acetone and oxygen are different, it is hard to compare the acetone oxidation activity between Cu0.50-NFs and catalysts in the literature precisely. However, Cu0.50-NFs does have excellent performance toward acetone oxidation, which can be comparable to Cu0.13Ce0.87Oy (ref. 37) and Ce-MSP.38 | ||
Fig. 7 Conversion of acetone as a function of reaction temperature (500 ppm acetone, 5% O2, N2 balance, GHSV = 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ml g−1 h−1). 000 ml g−1 h−1). | ||
According to the characteristic and catalytic performance results, the high activity of nanofiber catalysts can be attributed to the synergistic effect of specific nanofibrous morphology and abundant oxygen vacancies and cations with unusual oxidation states, which are both caused by the finely dispersed Cu species and formation of Cu–Ce–O solid solution. The reasons are addressed as follows.
Morphology characterization illustrated that the CuCeOx nanofiber catalysts possess one-dimensional nanofibrous morphology with a large specific surface area. As is well known, nanofibrous morphology favors the catalytic reaction because it can provide a large specific surface area33 and prevent the inhibition of the mass-transfer process,34 i.e. diffusion and adsorption/desorption of reactants/products. On the one hand, the large specific surface area is conductive to the dispersion of active phase and hence provides more active sites for reaction. On the other hand, the open structure composed by bonding the intersecting nanofibers facilitates the diffusion of gas molecules. In addition, the special nanofibrous morphology not only makes the adsorption of reactant toward the exterior or interior surfaces of nanofiber bundles easier, but also benefits the separation of product. Consequently, the mass-transfer process will be enhanced and the reactions can be accelerated. In conclusion, the nanofibrous morphology with large specific surface area is one of the pivotal factors governing the catalytic activity, and electrospinning is an appropriate method to obtain nanofibrous morphology which can provide large specific area.
It is widely known that during VOCs catalytic oxidation reactions over metal oxides, VOCs are firstly oxidized by the lattice oxygen of metal oxides, and then the latter is re-oxidized by the gas-phase oxygen.35,36 In this oxygen transfer cycle, two factors play the governing roles. One is oxygen vacancies as they can accelerate the dissociation of oxygen molecules on the surface and increase the mobility of lattice oxygen. The other is the unusual oxidation states of transition metal ions as a facile redox cycle can be formed between the cations with different valences. The results of XRD indicated the existence of highly dispersed Cu species and incorporated Cu species of Cu–Ce–O solid solution in nanofiber catalysts. These Cu species can lead to the substitution of Ce4+ with Cu2+ and structural defects of the CeO2 lattice, thus influence the electron balance of the lattice. In order to retain electroneutrality, there are two strategies: (a) the oxygen vacancies will increase and then some oxygen species will be adsorbed on the surface oxygen vacancies; therefore, the percentage of surface adsorbed oxygen in Cu0.50-NFs is more than that of Cu0.50-sol and Cu0.50-cmb. (b) The amount of unusual oxidation state cations will be enhanced. Therefore, the Ce3+ relative content of Cu0.50-NFs is more than that of Cu0.50-sol and Cu0.50-cmb. As mentioned above, the enhancement of oxygen vacancies and unusual oxidation state cations will improve the activity of the oxidation reaction; thus, T50 of Cu0.50-NFs is lower than that of Cu0.50-sol and Cu0.50-cmb.
4 Conclusions
CuCeOx nanofiber catalysts were successfully synthesized via electrospinning of alcohol/water solutions of Cu salt, Ce salt, and PVP. The catalytic activity test proved that the Cu0.50Ce0.50Ox nanofiber catalyst (NFs) has the highest activity (T50 = 190 °C) for acetone abatement among all these nanofiber catalysts. Moreover, this catalyst is much more active than Cu0.50Ce0.50Ox synthesized via sol–gel (T50 = 220 °C) and the urea-nitrate combustion method (T50 = 240 °C). The reason can be concluded as follows: (1) nanofibrous morphology with large specific surface area, (2) abundant oxygen vacancies and (3) cations with unusual oxidation states. Cu0.50-NFs possess the largest specific surface area (74.5 m2 g−1) among all nanofiber catalysts. Its specific surface area is about 3.7 and 31.3 times of those of the catalysts synthesized via sol–gel (sol) and the combustion (cmb) methods. XRD patterns showed Cu species disperse uniformly over all nanofiber catalysts, and the degree of metal oxide aggregation is weaker than that of Cu0.50-sol and Cu0.5-cmb. The decreased lattice parameter of cerium oxide suggests a large portion of Cu over nanofiber catalysts are incorporated into CeO2 lattice in the form of Cu–Ce–O solid solution, leading to structure defects and interface oxygen vacancies. The amount of surface adsorbed oxygen (Oads) and Ce3+ relative content in nanofiber catalysts vary with the increase of Cu/(Cu + Ce) molar ratio. According to XPS results, the Cu0.50-NF catalyst possesses the highest Oads percentage (52.4%) and Ce3+ relative content (18.38%), which indicates the high density of oxygen vacancies and abundant cations with unusual oxidation states. Thus, electrospinning is an effective method to prepare nanofiber CuCeOx catalysts with good acetone oxidation activity. This method can be extended to other metal oxide or noble metal catalysts for various catalytic reactions.Acknowledgements
This work was supported by the National Science Fund for Distinguished Young Scholars (no. 51125025) and the Key Innovation Team for Science and Technology of Zhejiang Province (Grant no. 2011R50017).References
- G. Zhou, H. Lan, X. Yang, Q. Du, H. Xie and M. Fu, Ceram. Int., 2013, 39, 3677–3683 CrossRef CAS PubMed.
- H. Huang, F. Haghighat and P. Blondeau, Indoor Air, 2006, 16, 236–247 CrossRef CAS PubMed.
- Y.-S. Chen and H.-S. Liu, Ind. Eng. Chem. Res., 2002, 41, 1583–1588 CrossRef CAS.
- T. Oda, J. Electrost., 2003, 57, 293–311 CrossRef CAS.
- C. Zheng, X. Zhu, X. Gao, L. Liu, Q. Chang, Z. Luo and K. Cen, J. Ind. Eng. Chem., 2013, 10, 2761–2768 Search PubMed.
- C. He, F. Zhang, L. Yue, X. Shang, J. Chen and Z. Hao, Appl. Catal., B, 2012, 111–112, 46–57 CrossRef CAS PubMed.
- G. C. Pontelli, R. P. Reolon, A. K. Alves, F. A. Berutti and C. P. Bergmann, Appl. Catal., A, 2011, 405, 79–83 CrossRef CAS PubMed.
- B. Solsona, M. Perez-Cabero, I. Vazquez, A. Dejoz, T. Garcia, J. Alvarez-Rodriguez, J. El-Haskouri, D. Beltran and P. Amoros, Chem. Eng. J., 2012, 187, 391–400 CrossRef CAS PubMed.
- S. Xu, D. Sun, H. Liu, X. Wang and X. Yan, Catal. Commun., 2011, 12, 514–518 CrossRef CAS PubMed.
- J. Sun, L. Bo, L. Yang, X. Liang and X. Hu, RSC Adv., 2014, 4, 14385–14391 RSC.
- A. A. Taha, A. A. Hriez, H. Wang, Y.-n. Wu and F. Li, RSC Adv., 2014, 4, 5901–5905 RSC.
- P. M. Heynderickx, J. W. Thybaut, H. Poelman, D. Poelman and G. B. Marin, J. Catal., 2010, 272, 109–120 CrossRef CAS PubMed.
- D. Delimaris and T. Ioannides, Appl. Catal., B, 2008, 84, 303–312 CrossRef CAS PubMed.
- D. Delimaris and T. Ioannides, Appl. Catal., B, 2009, 89, 295–302 CrossRef CAS PubMed.
- M. Raciulete and P. Afanasiev, Appl. Catal., A, 2009, 368, 79–86 CrossRef CAS PubMed.
- S. M. Saqer, D. I. Kondarides and X. E. Verykios, Appl. Catal., B, 2011, 103, 275–286 CrossRef CAS PubMed.
- X. Zhang, V. Thavasi, S. G. Mhaisalkar and S. Ramakrishna, Nanoscale, 2012, 4, 1707–1716 RSC.
- Y. Wang, Y. R. Su, L. Qiao, L. X. Liu, Q. Su, C. Q. Zhu and X. Q. Liu, Nanotechnology, 2011, 22, 225702 CrossRef CAS PubMed.
- C. He, J. Li, P. Li, J. Cheng, Z. Hao and Z.-P. Xu, Appl. Catal., B, 2010, 96, 466–475 CrossRef CAS PubMed.
- H. Li, G. Lu, Q. Dai, Y. Wang, Y. Guo and Y. Guo, Appl. Catal., B, 2011, 102, 475–483 CrossRef CAS PubMed.
- Y. Dai, W. Liu, E. Formo, Y. Sun and Y. Xia, Polym. Adv. Technol., 2011, 22, 326–338 CrossRef CAS PubMed.
- X. Wu, Q. Liang, D. Weng and Z. Lu, Catal. Commun., 2007, 8, 2110–2114 CrossRef CAS PubMed.
- A. Yousef, N. A. M. Barakat, T. Amna, S. S. Al-Deyab, M. S. Hassan, A. Abdel-hay and H. Y. Kim, Ceram. Int., 2012, 38, 4525–4532 CrossRef CAS PubMed.
- C. He, Y. Yu, L. Yue, N. Qiao, J. Li, Q. Shen, W. Yu, J. Chen and Z. Hao, Appl. Catal., B, 2014, 147, 156–166 CrossRef CAS PubMed.
- Z. Wang, G. Shen, J. Li, H. Liu, Q. Wang and Y. Chen, Appl. Catal., B, 2013, 138–139, 253–259 CrossRef CAS PubMed.
- W. Xingyi, K. Qian and L. Dao, Appl. Catal., B, 2009, 86, 166–175 CrossRef PubMed.
- L. Qi, Q. Yu, Y. Dai, C. Tang, L. Liu, H. Zhang, F. Gao, L. Dong and Y. Chen, Appl. Catal., B, 2012, 119, 308–320 CrossRef PubMed.
- L.-H. Chang, N. Sasirekha, Y.-W. Chen and W.-J. Wang, Ind. Eng. Chem. Res., 2006, 45, 4927–4935 CrossRef CAS.
- R. Qu, X. Gao, K. Cen and J. Li, Appl. Catal., B, 2013, 142, 290–297 CrossRef PubMed.
- H. He, H. Dai and C. Au, Appl. Catal., B, 2001, 33, 65–80 CrossRef CAS.
- A. Trovarelli and P. Fornasiero, Catalysis by ceria and related materials, World Scientific, 2013 Search PubMed.
- Z.-Q. Zou, M. Meng and Y.-Q. Zha, J. Phys. Chem. C, 2009, 114, 468–477 Search PubMed.
- H. Xiang, Y. Long, X. Yu, X. Zhang, N. Zhao and J. Xu, CrystEngComm, 2011, 13, 4856–4860 RSC.
- C. Pham-Huu, N. Keller, G. Ehret, L. c. J. Charbonniere, R. Ziessel and M. J. Ledoux, J. Mol. Catal. A: Chem., 2001, 170, 155–163 CrossRef CAS.
- V. Santos, M. Pereira, J. Órfão and J. Figueiredo, Appl. Catal., B, 2010, 99, 353–363 CrossRef CAS PubMed.
- S. C. Kim and W. G. Shim, Appl. Catal., B, 2010, 98, 180–185 CrossRef CAS PubMed.
- C. Hu, Q. Zhu, Z. Jiang, L. Chen and R. Wu, Chem. Eng. J., 2009, 152, 583–590 CrossRef CAS PubMed.
- C. Wang and H. Bai, Ind. Eng. Chem. Res., 2011, 50, 3842–3848 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |