A pyrene-functionalized Zinc(ii)–BPEA complex: sensing and discrimination of ATP, ADP and AMP†
Abstract
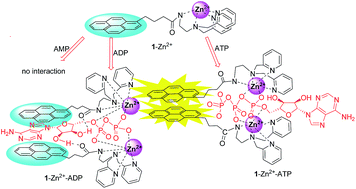
A tailor-made ratiometric fluorescent probe for nucleoside polyphosphates was fabricated based on a pyrene-functionalized zinc(II)–BPEA complex. The significant difference in both the fluorescence and 1H NMR signals of the probe on addition of ATP, ADP and AMP enables efficient discrimination between ATP, ADP and AMP.

 Please wait while we load your content...
Please wait while we load your content...