Polybutene-1 tube with in situ microfibering polystyrene via helical convergent flow: an economical pathway to continuously fabricate biaxially reinforced polyolefin tubes for medical application†
Abstract
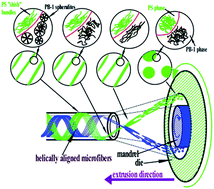
Polystyrene (PS) microfibers with high molecular orientation and helical alignment were in situ formed via helical convergent flow during polybutene-1 (PB-1) tubing processing to achieve performance improvements in both axial and hoop directions, which opened up an economical pathway for continuous fabrication of biaxially reinforced polyolefin tubes for medical application.

 Please wait while we load your content...
Please wait while we load your content...