Luminescent hybrid perovskite nanoparticles as a new platform for selective detection of 2,4,6-trinitrophenol†
Chinnadurai Muthu,
Sunena R. Nagamma and
Vijayakumar C. Nair*
Photosciences and Photonics Group, Chemical Sciences and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Academy of Scientific and Innovative Research (AcSIR), Trivandrum 695 019, India. E-mail: vcnair80@gmail.com
First published on 23rd October 2014
Abstract
Luminescent organic–inorganic perovskite (CH3NH3PbBr3) nanoparticles are used for the detection of 2,4,6-trinitrophenol (TNP, picric acid) in the solution and vapour state. Unlike most fluorescence based sensors, hybrid perovskite nanoparticles showed high selectivity and good sensitivity towards TNP, particularly in the solution state. Hydrogen bonding ability and electron accepting strength of TNP were found to play key roles in the detection mechanism.
Organic–inorganic hybrid perovskites are a unique class of materials with excellent optical and electrical properties.1 They can be represented by the general formula ABX3, where ‘A’ is an organic cation, ‘B’ is a metal cation and ‘X’ is a halide. They are versatile materials and can be easily prepared with low cost reagents and methods. Recently, hybrid perovskites attracted immense scientific attention due to their potential for making photovoltaic devices with high efficiencies.2 A number of hybrid perovskites exhibit excellent luminescence properties, which can be precisely tuned by simple modifications of their basic chemical structure.3 Since these materials possess advantages of both organic (excellent processability and mechanical properties) and inorganic (high ordering and electronic mobility) materials, they are expected to find applications in display and sensor devices also. Towards this end, very recently, Galian, Pérez-Prieto et al. reported a novel method for the synthesis of highly emissive perovskite nanoparticles and their application in light emitting diodes.4 However, the potential use of luminescent perovskites for sensor applications is not explored so far.
Nitroaromatics such as 2,4-dinitrotoluene (DNT), 2,4,6-trinitrotoluene (TNT) and 2,4,6-trinitrophenol (TNP, picric acid) are the most potent explosives used for making lethal weapons. Among the three, the explosive power of TNP is superior to the other two and it is a major component in many unexploded land mines present worldwide. TNP is also widely used in the manufacture of rocket fuels, fireworks, analytical reagents, photographic emulsions, etc. and presents in several industrial wastes. It contaminates soil, groundwater and plant foods, and is dangerous to wild life and human health because of its biological persistence, and toxicity.5 Though there are several materials available for the detection of explosives in general,6 surprisingly, less attention has been paid to the selective detection of TNP.7 Most of the reported materials for the detection of nitroaromatic explosives rely on fluorescence signalling, which in turn associated with the photoinduced electron transfer from the sensor (donor) to the analyte (acceptor).8 Since nitroaromatic explosives are excellent acceptors, they can effectively quench the fluorescence of any donor material resulting in poor selectivity. However, considering the adverse effects of TNP on environment and living beings, it is important to find new materials for the selective and sensitive detection of TNP. Herein, we report the use of a luminescent organic–inorganic hybrid perovskite (CH3NH3PbBr3) nanoparticles for the selective and sensitive detection of an explosive, TNP.
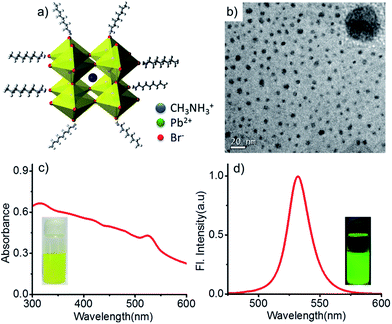
The CH3NH3PbBr3 nanoparticles having octylammonium bromide as capping agent (Fig. 1a) were prepared using the procedure reported by Galian, Pérez-Prieto et al.4 The nanoparticles were characterized by transmission electron microscopy (TEM), UV-Vis absorption spectroscopy, and fluorescence spectroscopy. TEM images showed spherical nanoparticles with an average diameter of 6.1 nm and relatively uniform dispersion (Fig. 1b; The size distribution of the nanoparticles is shown in Fig. S1 in the ESI†). They exhibited strong absorption spanning the entire UV-vis region with a maximum at 525 nm (Fig. 1c). Intense fluorescence with an emission maximum at 531 nm and a narrow full width at half-maximum of 21 nm was observed on excitation at 450 nm (Fig. 1d). The toluene suspension of the nanoparticles was yellow in color under room light and emitted bright green fluorescence on irradiation with 364 nm UV light as shown in the insets of Fig. 1c and d respectively.
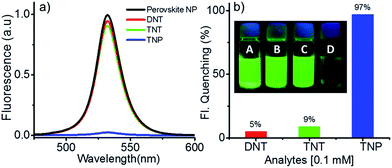
Ability of the perovskite nanoparticles to sense nitroaromatic explosives was analysed by adding DNT, TNT and TNP (50 μL) to the toluene suspension (3 mL) of the former. The solutions of the explosives were prepared in such a way that their concentration will be 0.1 mM in 3 mL of the perovskite suspension. Both DNT and TNT showed no appreciable quenching. On the other hand, TNP showed nearly complete quenching of the perovskite fluorescence almost instantaneously on addition. The emission spectrum of perovskite nanoparticles in the presence of DNT, TNT and TNP is shown in Fig. 2a. About 5 and 9% fluorescence quenching was seen in the presence of DNT and TNT, respectively. Under identical conditions, TNP exhibited around 97% quenching (Fig. 2b). Photographs of the corresponding solutions under 364 nm UV light is shown in the inset of Fig. 2b.
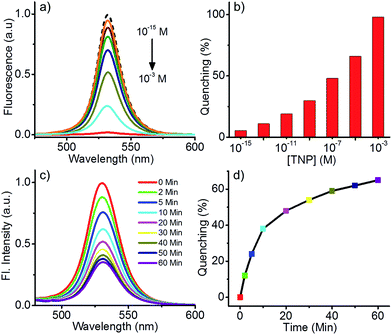
To find the minimum detection limit for TNP, fluorescence quenching studies with different concentrations of TNP (10−3 M to 10−15 M) was done (Fig. 3a and b). It was seen that noticeable change (∼6% quenching) in fluorescence was observed even at a concentration as low as femtomolar (10−15 M) of TNP. About 30% quenching was observed in the presence of nM concentrations of TNP. This observation clearly indicates that the perovskite nanoparticles can detect TNP with very high sensitivity.
Though the perovskite nanoparticles are capable of detecting TNP with excellent selectivity and high sensitivity in solution state, it is very important to detect it in the vapour state for practical applications. However, the vapour pressure of TNP (7.48 × 10−7 Torr at 25 °C) is 7 times lesser than that of TNT and 350 times lesser than that of DNT, make it hard to detect in the vapour phase.9 Nevertheless, the perovskite nanoparticles showed significant fluorescence quenching when exposed to TNP vapour (Fig. 3c). For this experiment, the nanoparticles were coated on a non-fluorescent filter paper strip and kept inside a vial containing saturated vapours of TNP. The fluorescence of the nanoparticles was monitored at different time intervals. About 12% quenching was observed within 2 minutes of exposure and a maximum of 65% quenching was observed in one hour. Changes in the fluorescence were negligible after one hour. The changes to the nanoparticle fluorescence with time on exposure to TNP vapours is shown in Fig. 3c, and the quenching % vs. time is shown in Fig. 3d. Quenching experiments with TNT and DNT in the vapour phase was also carried out under identical conditions. The studies revealed that the quenching efficiency follows the order TNP > DNT > TNT, but the selectivity between TNP and DNT was found to be less, which could be correlated to the higher vapour pressure (correspondingly a higher concentration) of the latter (see Fig. S2 in the ESI for details†).
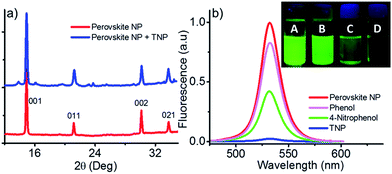
As mentioned earlier, the distinction between DNT, TNT and TNP is difficult using fluorescence based sensors because all of them are good electron acceptors and quenches the donor fluorescence efficiently. However, in the present case, high selectivity and sensitivity was observed for the detection of TNP, particularly in the solution state. We anticipated that the hydroxyl group in TNP plays a key role on the overall detection mechanism by interacting with the perovskite nanoparticles through hydrogen bonding. Since such interactions may result phase distortions to the perovskite structure, we monitored the powder XRD profile of the material in the absence and presence of TNP. The pristine nanoparticles exhibited sharp and intense peaks corresponding to 001 (d = 5.93 Å), 011 (d = 4.20 Å), 002 (d = 2.97 Å), 021 (d = 2.65 Å) planes4 (Fig. 4a). No change in the XRD profile was seen on addition of small amounts of TNP. However, at higher concentrations of TNP (∼1 mM), new peaks were started appearing in the XRD profile indicating phase distortions to the nanoparticle assembly. In order to get a better insight into the role of hydroxyl on fluorescence quenching, we have selected phenol and 4-nitrophenol, two aromatic compounds with hydroxyl group, as the analytes. As shown in Fig. 2a 0.1 mM of TNP quenches 97% of the fluorescence. On the other hand, under identical conditions, 4-nitrophenol and phenol can quench up to 54% and 19% of the fluorescence of the nanoparticles (Fig. 4b). Considering the quenching efficiency of the three analytes, it was clear that not only hydroxyl group but also the number of nitro groups on the analyte plays a key role on the quenching mechanism.
From the above study, it could be assumed that the hydroxyl group of TNP forms stable hydrogen bond with perovskite nanoparticles. This interaction could bring the analytes very close to the nanoparticles resulting in fluorescence quenching. Since the exciton diffusion and charge separation is very efficient in perovskite,10 electron transfer could be possible from perovskites to the analytes. This might be acting as the major pathway for the fluorescence quenching. This also explains the higher quenching efficiency for TNP (having three nitro groups, stronger acceptor) when compared to that of nitrophenol (having one nitro group, weaker acceptor). In addition to that, the hydrogen bonding interactions induce some phase distortions, especially at higher concentration of the analyte, resulting in minor quenching of the fluorescence. This could be attributed to the fluorescence quenching of the nanoparticles in the presence of phenol. Since DNT or TNT doesn't have any hydroxyl groups, there is no driving force for them to come closer to the nanoparticles resulting in poor quenching. This is particularly true in the solution state, where the sensor nanoparticles and analytes move continuously. Based on these observations, the sensing phenomenon could be schematically represented as shown in Fig. 5.
 | ||
| Fig. 5 Simplified schematic representation of the picric acid sensing mechanism by perovskite nanoparticles. | ||
In summary, fluorescent nanoparticles derived from an organic–inorganic hybrid perovskite (CH3NH3PbBr3) can be used as an excellent sensor for the detection of TNP with high selectivity and sensitivity. The sensitivity and selectivity of this material is comparable with that of several organic molecules and polymers reported in the literature.11 Studies revealed that the presence of hydrogen bonding hydroxyl group and nitro groups plays a key role on the detection mechanism. Moreover, the present work illustrates the potential of hybrid perovskites for sensor applications, which is a very important area similar to that of photovoltaics.
Acknowledgements
We thank CSIR network project CSC0134 for financial support. V.C.N. thanks DST for Ramanujan Fellowship. C.M. is grateful to CSIR for Junior Research Fellowship. We acknowledge Kiran Mohan and Robert Philip for TEM analysis, Bhoje Gowd for XRD measurement and Kiran J. S. for artwork.Notes and references
- (a) D. B. Mitzi, C. A. Feild, W. T. A. Harrison and A. M. Guloy, Nature, 1994, 369, 467 CrossRef CAS; (b) S. Kazim, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed., 2014, 53, 2812 CrossRef CAS PubMed; (c) Z. Cheng and J. Lin, CrystEngComm, 2010, 12, 2646 RSC.
- (a) H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J. E. Moser, M. Grätzel and N.-G. Park, Sci. Rep., 2012, 2, 591 Search PubMed; (b) M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395 CrossRef CAS PubMed.
- (a) E. R. Dohner, E. T. Hoke and H. I. Karunadasa, J. Am. Chem. Soc., 2014, 136, 1718 CrossRef CAS PubMed; (b) C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019 CrossRef CAS PubMed; (c) C. Wehrenfennig, M. Liu, H. J. Snaith, M. B. Johnston and L. M. Herz, J. Phys. Chem. Lett., 2014, 5, 1300 CrossRef CAS.
- L. C. Schmidt, A. Pertegás, S. González-Carrero, O. Malinkiewicz, S. Agouram, G. M. Espallargas, H. J. Bolink, R. E. Galian and J. Pérez-Prieto, J. Am. Chem. Soc., 2014, 136, 850 CrossRef CAS PubMed.
- J. F. Wyman, M. P. Serve, D. W. Hobson, L. H. Lee and D. E. Uddin, J. Toxicol. Environ. Health, Part A, 1992, 37, 313 CAS.
- (a) A. Rose, Z. Zhu, C. F. Madigan, T. M. Swager and V. Bulović, Nature, 2005, 434, 876 CrossRef CAS PubMed; (b) S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC; (c) K. K. Kartha, S. S. Babu, S. Srinivasan and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 4834 CrossRef CAS PubMed; (d) C. Vijayakumar, G. Tobin, W. Schmitt, M.-J. Kim and M. Takeuchi, Chem. Commun., 2010, 46, 874 RSC.
- (a) Y. Peng, A.-J. Zhang, M. Dong and Y.-W. Wang, Chem. Commun., 2011, 47, 4505 RSC; (b) B. Gole, S. Shanmugaraju, A. K. Bar and P. S. Mukherjee, Chem. Commun., 2011, 47, 10046 RSC; (c) V. Vij, V. Bhalla and M. Kumar, ACS Appl. Mater. Interfaces, 2013, 5, 5373 CrossRef CAS PubMed; (d) B. Xu, X. Wu, H. Li, H. Tong and L. Wang, Macromolecules, 2011, 44, 5089 CrossRef CAS.
- (a) J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864 CrossRef CAS; (b) T. Naddo, Y. Che, W. Zhang, K. Balakrishnan, X. Yang, M. Yen, J. Zhao, J. S. Moore and L. Zang, J. Am. Chem. Soc., 2007, 129, 6978 CrossRef CAS PubMed.
- H. Östmark, S. Wallin and H. G. Ang, Propellants, Explos., Pyrotech., 2012, 37, 12 CrossRef.
- (a) S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341 CrossRef CAS PubMed; (b) G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Grätzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344 CrossRef CAS PubMed.
- (a) M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543 RSC; (b) Y. Salinas, R. Martinez-Manez, M. D. Marcos, F. Sancenon, A. M. Costero, M. Parra and S. Gil, Chem. Soc. Rev., 2012, 41, 1261 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Procedure for synthesis, photophysical properties and description of experimental techniques. See DOI: 10.1039/c4ra07884e |
| This journal is © The Royal Society of Chemistry 2014 |