Optimization of process variables for supercritical liquefaction of giant fennel
Abstract
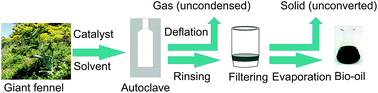
Milled giant fennel (Ferula orientalis L.) stalks were treated in supercritical solvents in the presence of catalyst in a high pressure reactor. Effects of process variables including temperature (from 240 to 320 °C), solvent (2-propanol, 2-butanol, and acetone), catalyst (Na2CO3, NaOH, and ZnCl2), particle size (from 0.224 > Dp > 0.150 to 0.850 > Dp > 0.425), solvent/mass ratio (from 50/5 to 50/15) and reaction time (from 45 to 95 min) on product yields were investigated. The amounts of solid, liquid and gas produced, as well as the properties of the resulting bio-oils were determined. Temperature, catalyst and reaction time were major factors affecting the product yields and composition of bio-oils. The highest conversion (liquid + gas products) of 73.48% was achieved in acetone with 10% zinc chloride at 320 °C. Acetone as solvent, zinc chloride (10%) as catalyst, 0.224 > Dp > 0.150 as particle size, 50/5 as solvent/mass ratio, and 80 minutes as reaction time provide the optimum conditions for the supercritical liquefaction of Ferula orientalis L. The liquid products (bio-oils) obtained at 300 °C were analyzed by gas chromatography-mass spectrometry (GC-MS). The bio-oils which contained a higher amount of carbon and hydrogen than that of the original raw material had higher heating values ranging from 23.66 to 26.17 MJ kg−1.

 Please wait while we load your content...
Please wait while we load your content...