Chemodosimeter for fluoride ions based on F−-triggered Si–O cleavage followed by the deprotonation/autoxidation of secondary nitrile C–H group†
Baiyun Li,
Chuanxiu Zhang,
Chuanxiang Liu*,
Jinju Chen,
Xinyu Wang,
Zhenjiang Liu and
Fengping Yi
School of Chemical and Environmental Engineering, Shanghai Institute of Technology, 201418 Shanghai, P. R. China. E-mail: cxliu@sit.edu.cn
First published on 16th September 2014
Abstract
A completely new strategy for a chemodosimeter for fluoride based on F−-triggered Si–O bond cleavage, followed by the deprotonation/autoxidation of secondary nitrile C–H group was developed for the first time. The chemodosimeter exhibited high selectivity and sensitivity, dramatic color “turn-on”, and fluorescence “turn-off” for the detection for fluoride.
Fluoride (F−) ions have attracted much interest because of their important roles in biological and chemical sciences.1 The over-intake of fluoride may cause osteoporosis and apoptosis in mammalian cells.2 Therefore, the design and development of artificial receptor-based sensors for the selective detection of F− is a challenging task in supramolecular chemistry.3 Colorimetric and fluorescent sensors with advantages such as no equipment requirement, simple detection by naked eye, high sensitivity, and low detection limits are more promising.4 Apart from the conventional hydrogen bonding and the deprotonation of labile protons, one of the emerging strategies is the development of reaction-based chemodosimeters for F−.5 In particular, the most intriguing approaches, which were mainly based on the Si–O bond cleavage using the great affinity of F− for silicon,6 have been investigated in details. These methods usually show specific selectivity for F−, and some of them are applicable in polar solvent or aqueous environments.7 However, the sensors with small absorption shift sometimes lack sensitivity as most of them require several minutes to even hours to generate a signal response. Therefore, the development of more effective chemodosimeters for F− ions overcoming these limitations is still challenging.
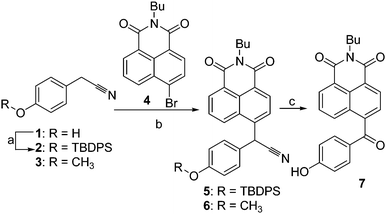
In our endeavor to develop highly sensitive chemodosimeters for F−,8 a mechanism involving the deprotonation/autoxidation of diaryl secondary nitriles was developed.9 To improve the sensitivity of the conventional hydrolysis of silyl ethers, we planned to investigate a dual reaction-based site utilized by the F−-triggered Si–O bond cleavage, followed by the deprotonation/autoxidation of the secondary nitrile group. To the best of our knowledge, this is a completely new sensing mechanism for F− compared to only F−-triggered Si–O bond cleavage. Based on the mechanism, we designed dosimeter 5 based on a 1,8-naphthalimide system containing a tert-butyldiphenylsilyl (TBDPS) moiety combined with a secondary nitrile CH group, together with the reference compounds 6 and 7 for comparison (Scheme 1). First, 2-(4-(tert-butyldiphenylsilyloxy)phenyl)acetonitrile (2, Fig. S1†) was prepared in 98% yield using a classical hydroxyl-protected procedure,10 in which the hydroxyl moiety of the corresponding 2-(4-hydroxyphenyl)acetonitrile 1 was transformed to the corresponding silyl ether using tert-butyldiphenylsilyl chloride (TBDPSCl). Further, the NaH-catalyzed condensation of N-butyl-4-bromo-1,8-naphthalimide 4 with intermediate 2 and 2-(4-methoxyphenyl)acetonitrile 3 afforded dosimeter 5 and reference compound 6 in 51% and 92% yields, respectively.9 Dosimeter 5 was desilylated and oxidatively decyanated with tetrabutylammonium fluoride (TABF) to afford compound 7 for comparison. Compounds 5–7 were characterized by 1H-NMR, 13C-NMR, IR, and HRMS-ESI analyses (Fig. S2–S13†).
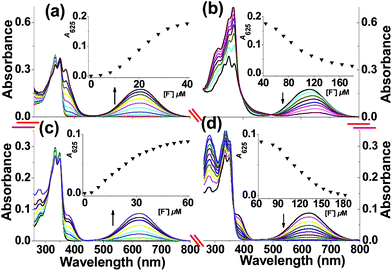
First, the UV-visible spectra of receptors 5 and 6 with increasing concentrations of F− (as its TBA salt) were investigated in acetonitrile (Fig. 1). Upon the addition of 0.0–2.0 equiv. of F−, two new bands centered at 370 and 625 nm appeared in the UV-visible spectra of dosimeter 5 (Fig. 1a). The appearance of a blue color and a larger red shift (≈275 nm) indicate the major formation of [C–H⋯F−] complexes.9 On further addition of F−, the intensity of the band at 625 nm decreased and slightly red-shifted to 660 nm (the color of the solution turned light brown), accompanied by a strong increasing band centered at 367 nm with isosbestic points at 390 and 500 nm (Fig. 1b). The strong peak at 367 nm was further verified by the comparison of the UV-visible spectra of reference compound 7 with increasing concentrations of F− (Fig. S14†), indicating the formation of phenolate anions because of the cleavage of the TBDPS group, followed by the oxidation of the C–H group to a rigid carbonyl group. Compared to compound 6 without Si–O group, a highly similar spectroscopic response was observed, as shown in Fig. 1c; however, as shown in Fig. 1d, the peak at 625 nm significantly decreased without red shift. Moreover, the saturated amounts of F− at 625 nm were different for dosimeters 5 and 6, in which dosimeter 5 containing a dual binding site was saturated by adding only 2.0 equiv. of F−. Thus, the sensitivity of 5 was significantly higher than that of the dosimeter containing only a C–H group (3.0 equiv. for 6). Furthermore, when more F− was added (2.0–9.0 equiv. F−), the slightly red-shifted peak at 660 nm for dosimeter 5 was ascribed to the formation of a phenolate anion with a strong negatively charged donor oxygen atom, to further enhance the potential intramolecular charge-transfer state of the [C–H⋯F−] complexes.11
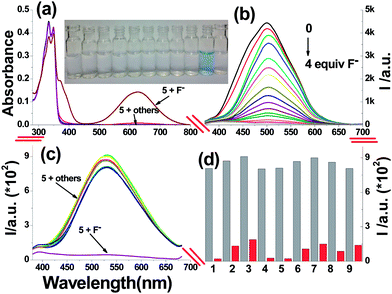
Next, the selectivity of dosimeter 5 for F− was investigated by UV-visible spectroscopy by adding various anions (AcO−, I−, Br−, BF4−, Cl−, NO3−, ClO4−, H2PO4−, HSO4− and F− ions, as the TBA salts) to 5 in acetonitrile (Fig. 2a). When TBAF (2.0 equiv.) was added to a solution of 5, two new peaks at 625 and 370 nm were observed in the UV-visible spectrum of dosimeter 5 accompanied by an instantaneous color change from colorless to blue (Fig. 2a, inset, naked-eye detection), whereas no considerable change was observed in the absorption spectra by adding other anions. These results indicate that dosimeter 5 shows excellent selectivity for F− over other anions (for the interference experiments by UV-visible spectroscopy, see: Fig. S15†). Moreover, a specific emission response to F− in acetonitrile was observed (Fig. 2b). Upon the addition of F− ions to the solution of 5, a significant decrease in the fluorescence intensity at 500 nm (λex = 350 nm) was observed, indicating that the photoinduced electron transfer (PET) was enhanced by an increased negative charge on the phenolate oxygen atom and a potential conjugate effect induced by the oxidation of diaryl secondary nitriles C–H group. Importantly, a linear fluorescence response to F− ions concentration in the range 0–40 μM (Fig. S16,† R2 = 0.9925) was observed, indicating that dosimeter 5 has great potential for the quantitative determination of F− ions. The corresponding detection limits in the fluorescence mode was determined to be 0.105 μM (Fig. S17†). Subsequently, AcO−, I−, Br−, BF4−, Cl−, NO3−, ClO4−, H2PO4−, HSO4− and F− ions (as their TBA salts) were used to measure the selectivity of dosimeter 5 in a mixture of acetonitrile–water (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v), and the fluorescence spectra were recorded after 10 min upon addition of 40.0 equiv. of each of these anions (Fig. 2c, in acetonitrile, see: Fig. S18†). Compared to the other anions investigated, only F− exhibited a remarkable fluorescence quenching at 530 nm, and the corresponding fluorescence titration of dosimeter 5 with F− ions in acetonitrile–water (95
5, v/v), and the fluorescence spectra were recorded after 10 min upon addition of 40.0 equiv. of each of these anions (Fig. 2c, in acetonitrile, see: Fig. S18†). Compared to the other anions investigated, only F− exhibited a remarkable fluorescence quenching at 530 nm, and the corresponding fluorescence titration of dosimeter 5 with F− ions in acetonitrile–water (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) was also recorded, as shown in Fig. S19.† This excellent selectivity was further highlighted by the interference experiments (Fig. 2d), in which a consistent turn-off fluorescent response was observed upon the addition of 40.0 equiv. of fluoride ions to the solutions of 5 containing equal concentrations of potentially competing anions (in acetonitrile, see: Fig. S20†). Dosimeter 5 might be selective based on anion basicity, and this could also be further confirmed by the UV-visible titration of dosimeter 5 as well as the reference compound 6 with the OH− ions (Fig. S21–S22†).
5, v/v) was also recorded, as shown in Fig. S19.† This excellent selectivity was further highlighted by the interference experiments (Fig. 2d), in which a consistent turn-off fluorescent response was observed upon the addition of 40.0 equiv. of fluoride ions to the solutions of 5 containing equal concentrations of potentially competing anions (in acetonitrile, see: Fig. S20†). Dosimeter 5 might be selective based on anion basicity, and this could also be further confirmed by the UV-visible titration of dosimeter 5 as well as the reference compound 6 with the OH− ions (Fig. S21–S22†).
To investigate the interactions between dosimeter 5 and F−, 1H-NMR titration experiments were performed in DMSO-d6. The addition of increasing concentration of F− ions results in the most remarkable upfield shift of the phenyl proton peaks “Ha′ and Hb′”. These results are in agreement with the formation of phenolate anion by F−-triggered silyl ether hydrolysis. At a higher F− ions concentration, the “Ha′ and Hb′” peaks were assigned to the broad peak at δ 6.0 ppm (red, #, Fig. 3), and a set of peaks (red, #) was highly overlapped with the peaks observed (*, blue) in final titration of reference 7 with F− ions. Meanwhile, the LC-MS analysis was further conducted to confirm the mechanism (Fig. S23†), and which clearly indicates the formation of compound phenolate 7 (calcd for 372 for C23H18NO4). Therefore, the mechanism base on F−-triggered Si–O cleavage followed by the deprotonation/autoxidation of a secondary nitrile C–H group is finally established.
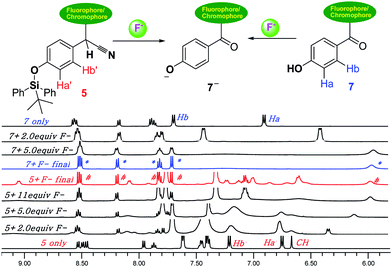 | ||
| Fig. 3 1H-NMR titration spectra of 5 (bottom, 30 mM) and 7 (top, 30 mM) in DMSO-d6 upon addition of F− ions (as tetrabutylammonium salts in DMSO-d6) at 298 K. | ||
In conclusion, we developed a new sensing mechanism for F− based on a dual reaction-based binding site including F−-triggered Si–O cleavage, followed by the deprotonation/autoxidation of a secondary nitrile C–H group. The dosimeter 5 exhibited high sensitivity and selectivity as well as naked-eye and fluorescent detection of F− in aqueous environments. Further studies on the development of novel chemodosimeters for F− ions using this strategy are in progress.
Acknowledgements
We thank the National Natural Science Foundation of China (Grant no. 21202099, 21102093) and the Opening Fund of Shanghai Key Laboratory of Chemical Biology (no. SKLCB-2013-05) for financial support.Notes and references
- (a) Supramolecular Chemsitry for Anions, ed. K. Binachi, K. Bowman-James and E. Garcia-Espana, New York, 1997 Search PubMed; (b) J.-M. Lehn, Supramolecular Chemistry, Concept and Perspectives, VCH, Weinheim, 1995 Search PubMed.
- (a) K. L. Kirk, Biochemsitry of the Elemental Halogens and Inorganic Halides, Plenum Press, New York, 1991 Search PubMed; (b) B. L. Riggs, Bone and Mineral Research, Annual 2, Elsevier, Amsterdam, 1984 Search PubMed; (c) T. J. Cheng, T. M. Chen, C.-H. Chen and Y. K. Lai, J. Cell. Biochem., 1998, 69, 221 CrossRef CAS.
- (a) J. L. Sessler and J. M. Davis, Acc. Chem. Res., 2001, 34, 989 CrossRef CAS; (b) P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef CAS; (c) R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Krugerc and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936 RSC; (d) P. A. Gale, Chem. Soc. Rev., 2010, 39, 3746 RSC; (e) J. L. Sessler, P. A. Gale and W. S. Cho, Anion Receptor Chemistry, RSC Pub, Cambridge, 2006 Search PubMed; (f) P. A. Gale, N. Busschaert, C. J. E. Haynes, L. E. Karagiannidis and I. L. Kirby, Chem. Soc. Rev., 2014, 43, 205 RSC.
- (a) M. E. Moragues, R. Martínez-Máñez and F. Sancenón, Chem. Soc. Rev., 2011, 40, 2593 RSC; (b) V. Amendola, D. Esteban-Gomez, L. Fabbrizzi and M. Licchelli, Acc. Chem. Res., 2006, 39, 343 CrossRef CAS PubMed; (c) J. Du, M. Hu, J. Fan and X. Peng, Chem. Soc. Rev., 2012, 41, 4511 RSC; (d) R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419 CrossRef PubMed; (e) Y. Hua and A. H. Flood, Chem. Soc. Rev., 2010, 39, 1262 RSC.
- (a) Y. Zhou, J. Zhang and J. Yoon, Chem. Rev., 2014, 114, 5511 CrossRef CAS PubMed; (b) C. R. Wade, A. E. J. Broomsgrove, S. Aldridge and F. P. Gabbai, Chem. Rev., 2010, 110, 3958 CrossRef CAS PubMed.
- (a) T.-H. Kim and T. M. Swager, Angew. Chem., Int. Ed., 2003, 42, 4803 CrossRef CAS PubMed; (b) J. Cao, C. Zhao and W. Zhu, Tetrahedron Lett., 2012, 53, 2107 CrossRef CAS; (c) G. Wei, J. Yin, X. Ma, S. Yu, D. Wei and Y. Du, Anal. Chim. Acta, 2011, 703, 219 CrossRef CAS PubMed; (d) J. F. Zhang, C. S. Lim, S. Bhuniya, B. R. Cho and J. S. Kim, Org. Lett., 2011, 13, 1190 CrossRef CAS PubMed; (e) P. Sokkalingam and C.-H. Lee, J. Org. Chem., 2011, 76, 3820 CrossRef CAS PubMed; (f) O. A. Bozdemir, F. Sozmen, O. Buyukcakir, R. Guliyev, Y. Cakmak and E. U. Akkaya, Org. Lett., 2010, 12, 1400 CrossRef CAS PubMed; (g) X. Cao, W. Lin, Q. Yu and J. Wang, Org. Lett., 2011, 13, 6098 CrossRef CAS PubMed; (h) Y. Bao, B. Liu, H. Wang, J. Tian and R. Bai, Chem. Commun., 2011, 47, 3957 RSC; (i) M. Park, D. Jang, S. Y. Kim and J.-I. Hong, New J. Chem., 2012, 36, 1145 RSC; (j) J. Cao, C. Zhao, P. Feng, Y. Zhang and W. Zhu, RSC Adv., 2012, 2, 418 RSC.
- (a) S. Y. Kim and J.-I. Hong, Org. Lett., 2007, 9, 3109 CrossRef CAS PubMed; (b) S. Y. Kim, J. Park, M. Koh, S. B. Park and J.-I. Hong, Chem. Commun., 2009, 4735 RSC; (c) R. Hu, J. Feng, D. Hu, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2010, 49, 4915 CrossRef CAS PubMed; (d) B. Zhu, F. Yuan, R. Li, Y. Li, Q. Wei, Z. Ma, B. Du and X. Zhang, Chem. Commun., 2011, 47, 7098 RSC; (e) D. Kim, S. Singha, T. Wang, E. Seo, J. H. Lee, S. J. Lee, K. H. Kim and K. H. Ahn, Chem. Commun., 2012, 48, 10243 RSC; (f) S. Elsayed, A. Agostini, L. E. Santos-Figueroa, R. Martínez-Máñez and F. Sancenón, ChemistryOpen, 2013, 2, 58 CrossRef CAS PubMed.
- (a) C. Liu, X. Qian, G. Sun, L. Zhao and Z. Li, New J. Chem., 2008, 32, 472 RSC; (b) C. Liu, X. Qian, J. Wang and Z. Li, Tetrahedron Lett., 2008, 49, 1087 CrossRef CAS.
- J. Chen, C. Liu, J. Zhang, W. Ding, M. Zhou and F. Wu, Chem. Commun., 2013, 49, 10814 RSC.
- E. S. Tan, M. Miyakawa, J. R. Bunzow, D. K. Grandy and T. S. Scanlan, J. Med. Chem., 2007, 50, 2787 CrossRef CAS PubMed.
- V. Amendola, G. Bergamaschi, M. Boiocchi, L. Fabbrizzi and L. Mosca, J. Am. Chem. Soc., 2013, 135, 6345 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis procedures, additional spectra and experimental details. See DOI: 10.1039/c4ra07870e |
| This journal is © The Royal Society of Chemistry 2014 |