An N-heterocyclic carbene-catalyzed approach to the indirect Friedländer quinoline synthesis†
Yanfang Zhu and
Chun Cai*
Chemical Engineering College, Nanjing University of Science & Technology, 200 Xiaolingwei, Nanjing 210094, P. R. China. E-mail: c.cai@mail.njust.edu.cn; Fax: +86-25-84315030; Tel: +86-25-84315514
First published on 15th October 2014
Abstract
Quinolines have been obtained through the indirect Friendländer annulation starting from 2-aminobenzyl alcohol or derivatives from it and ketones catalyzed by N-heterocyclic carbene, and the synthesis of polysubstituted quinolines through a one-pot, two-step tandem reaction starting from readily available ketones and alcohols via alpha-alkylation and indirect Friedländer annulation under air also has been presented.
The quinoline scaffold is one of the ubiquitous structures that exist in pharmaceuticals and biologicals.1 2-arylquinoline scaffolds are of particular importance for the construction of a wide range of biologically active molecules, such as P-selectin antagonism, antimalarial, and antitumor activities.2–6 Because of the diverse pharmacological value of quinolines, a variety of novel and expeditious approaches have been reported in recent years.
Conventional routes for the synthesis of quinolines, such as the Skraup, Doebner–von Miller, Conrad–Limpach, and Pfitzinger syntheses, suffer from harsh reaction conditions, low stereoselectivity or consist of multiple steps, resulting in low overall yields, limiting their applicability.7 The Friendländer annulation starting from unstable 2-aminobenzaldehyde and ketones has proven to be one of the most simple, straightforward, and widely used ways to develop quinoline compounds. Despite some acids8–16 have been employed for the reaction, these procedures often suffer from the unstable 2-aminobenzaldehyde17 and the formation of side products as a result of the self-aldol condensation of the 2-aminoaryl carbonyls.18
In order to overcome the drawbacks mentioned before, the transition-metal catalyzed the indirect Friedländer annulation has obtained considerable attention as a useful tool for the synthesis of quinoline compounds, in which the indirect Friedländer reaction using 2-aminobenzyl alcohols instead of 2-aminoaryl carbonyls have emerged as a promising alternative.19–21 However, this indirect method gave final products contaminated with traces of transition metals, which are limited in some industrial applications. Otherwise, base-mediated indirect Friedländer transformations can also afford quinoline compounds. In 2008, Yus and co-workers17 reported the t-BuOK (1.0 equiv.) catalyzed system with benzophenone (100 mol%) as hydride scavenger in dioxane at 90 °C under an argon atmosphere gave the expected 2-phenyl quinoline in an excellent yield (99%). In the same year, Verpoort and co-workers22 demonstrated the same example in dioxane using t-BuOK (1.5 equiv.) as the catalyst at 80 °C under air with the yield of 94%. And Liang et al.23 reported this reaction proceed in toluene with t-BuOLi (2.0 equiv.) as the catalyst at 110 °C under an argon atmosphere afforded the yield of 94% after 12 h. However, these approaches often suffer from excess bases, high temperature, and sometimes need an atmosphere of argon. Therefore, development of an alternative method for the indirect Friedländer transformations still remains a challenge.
In recent years, N-heterocyclic carbenes (NHCs) have received considerable attention as an important and powerful class of organocatalysts24–26 with tremendous applications in a variety of synthetic transformations and as versatile ligands27–29 in transition metal catalysis. However, the reaction catalyzed by NHCs employing other substrates was quite limited,30–32 except aldehydes.33–39 Herein, we wish to report the indirect Friedländer synthesis of quinolines from aminoalcohol and ketone using NHCs in the presence of a base, without any transition-metal catalyst. (Scheme 1).
 | ||
| Scheme 1 Synthesis of substituted quinolines from ketones and 2-aminobenzyl alcohol or derivatives from it. | ||
Our investigation began with the reaction of 2-aminobenzyl alcohol 1a with acetophenone 2a catalyzed by precursor A in the presence of KOH under air at 60 °C. To our delight, the target annulation product 3a was obtained (Table 1, entry 1). Consequently, a series of other precursors (Fig.1) were evaluated, wherein precursor B displayed the highest catalytic activity (Table 1, entry 2). Optimal amount of acetophenone 2a was selected to be two equivalents to 1a (Table 1, entries 2 and 5). The excess ketone may serve as a hydrogen acceptor, accelerating the oxidation of the alcohol function to an aldehyde. It was found that the bases drastically affected the reaction. An excellent yield (90%) of product 3a can be achieved when KOH was used (Table 1, entry 2), while, using other weaker bases such as K2CO3, NEt3 and Cs2CO3, no reaction occurred (Table 1, entries 6–8). In addition, decreased base dosage was found to afford unsatisfactory yield of 3a (Table 1, entry 9). With the amount of precursor B decreased to 1 mol%, the yield dropped to 80% (Table 1, entry 10). Optimization of solvents for the synthesis of 3a employing the precursor B was also undertaken and it was found that among toluene, DMF, CH3Cl and dioxane (Table 1, entries 2, 11–13), the best solvent in terms of yield was toluene (Table 1, entry 2). Thus, nonpolar solvents, toluene, chloromethane were proven to be better effective than polar solvents on the reaction. Notably, performing the reaction in the absence of precursor B led to a low yield product 3a (Table 1, entry 14). It should be noted that the indirect Friedländer synthesis of quinolines from aminoalcohol and ketones using NHC catalyst could be proceed readily at a lower temperature and under an atmosphere of air, as compared to those approaches that only using base as catalysts.17,22,23
| Entry | Precursor | 1a:2a | Base (equiv.) | Solvent | Isolated yield [%] |
|---|---|---|---|---|---|
| a Reaction conditions: 1a, 2a and precursor (2 mol%) were mixed together in toluene (2 ml) under air and finally base was added, 60 °C, for 1 h.b Precursor (1 mol%) was used. | |||||
| 1 | A | 1:2 | KOH(1) | Toluene | 84 |
| 2 | B | 1:2 | KOH(1) | Toluene | 90 |
| 3 | C | 1:2 | KOH(1) | Toluene | 79 |
| 4 | D | 1:2 | KOH(1) | Toluene | 82 |
| 5 | B | 1:1 | KOH(1) | Toluene | 61 |
| 6 | B | 1:2 | K2CO3(1) | Toluene | nr |
| 7 | B | 1:2 | NEt3(1) | Toluene | nr |
| 8 | B | 1:2 | Cs2CO3(1) | Toluene | nr |
| 9 | B | 1:2 | KOH(0.5) | Toluene | 69 |
| 10b | B | 1:2 | KOH(1) | Toluene | 80 |
| 11 | B | 1:2 | KOH(1) | DMF | 43 |
| 12 | B | 1:2 | KOH(1) | CH3Cl | 85 |
| 13 | B | 1:2 | KOH(1) | Dioxane | 38 |
| 14 | — | 1:2 | KOH(1) | Toluene | 38 |
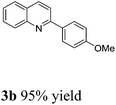
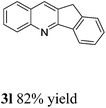
With the optimized reaction conditions in hand, we set out to explore the substrate scope of this process. In all cases, moderate to excellent yields could be achieved. Both electron-donating substituents including methyl and methoxy groups as well as electron-withdrawing substituents such as bromo and trifluoromethyl groups on the aromatic ring of acetophenone were well tolerated in this reaction (Table 2, 3a–3e). The quinolines using other aryl ketones with longer chains also could be obtained in excellent yields (3f, 3g in Table 2). In addition, the protocol could be employed also with aliphatic ketones, with similar excellent results (3h–3l in Table 2). 2-Acetylpyridine was well tolerated in this reaction to give 3m in moderate yield. 2-Aminobenzyl alcohols with methyl on the alpha-position also finished the desired products 3n in good yield. 2-Aminobenzyl alcohol with electron-donating and electron-withdrawing groups on the aromatic ring gave excellent yields of products (3o, 3p in Table 2).
| a Reaction conditions: 1 (0.5 mmol), 2 (1.0 mmol), precursor B (2 mol%), KOH (0.5 mmol), toluene (2 mL), 60 °C, for 1 h, under air. Isolated yield based on 2-aminobenzyl alcohol. | ||
|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
||
Encouraged by upon results we then envisioned that the use of alpha-alkylation of ketones with alcohols in conjunction with the indirect Friedländer synthesis would be suitable for creating more complicated quinoline compounds. To our delight, this reaction proceeded very smoothly, afforded the corresponding products in moderate to excellent yields. When 4a was reacted with 5a in the presence of 0.2 mol% of precursor A and 1.0 equiv. of KOH at 110 °C (bath temp) for 5 h, the desired α-alkylated ketone 6a was formed in 98% yield. For the second step, substrates 6a and 2-aminobenzyl alcohol were reacted at 60 °C for 1 h with the precursor B and 1 equiv. of KOH to form the product quinoline 7a in 46% yield. When 6a was allowed to react with 2-aminobenzyl alcohol at 80 °C for 4 h, 7a was produced in 85% yield. After establishing ideal conditions for the one-pot reaction, we investigated the scope of this two-step tandem reaction with various ketones and alcohols catalyzed by NHC. The electronic effect had an obvious influence on this reaction (Table 3, 7a–7e). Electron-rich benzyl ketones and benzyl alcohols have a relatively higher reactivity, affording the corresponding products in good yields (Table 3, 7b, 7d). 2-Aminobenzyl alcohol with electron-withdrawing groups on the aromatic ring and methyl on the alpha-position also finished the desired products in good yields (Table 3, 7f, 7g). It should be noted that the indirect Friedländer synthesis of quinolines through a one-pot, two-step tandem reaction using NHC catalyst could be proceed readily at a lower temperature and under air, as compared to the approach.21
| Entry | R1 | R2 | R5 | R6 | Quinoline | Isolated yield [%] |
|---|---|---|---|---|---|---|
| a Reaction conditions: 5 (0.6 mmol), 4 (0.5 mmol), precursor A (0.2 mol%), KOH (0.5 mmol), toluene (1.5 mL), 110 °C, 5 h. This was followed by the addition of 1 (0.25 mmol), precursor B (2 mol%), KOH (0.25 mmol), 80 °C, 4 h.b Reaction conditions: 5 (0.6 mmol), 4 (0.5 mmol), precursor A (0.2 mol%), KOH (0.5 mmol), toluene (1.5 mL), 110 °C, 5 h. This was followed by the addition of 2-aminobenzyl alcohol (0.25 mmol), precursor B (2 mol%), KOH (0.25 mmol), 60 °C, 4 h. | ||||||
| 1 | H | H | H | H | 7a | 85 (46)b |
| 2 | H | H | H | OMe | 7b | 83 |
| 3 | H | H | H | Br | 7c | 65 |
| 4 | H | H | OMe | H | 7d | 81 |
| 5 | H | H | CF3 | H | 7e | 63 |
| 6 | H | CH3 | H | H | 7f | 68 |
| 7 | Br | H | H | H | 7g | 72 |
The proposed mechanism for the reaction is presented in Scheme 2. The base deprotonates N-heterocyclic carbene salt to generate a free carbene. The role of NHC may be to assist both proton and hydride transfer from 1 to 2, forming the intermediate. One equivalent of 2 acts as hydrogen acceptor and is converted to the corresponding alcohol in the oxidation process of 1. A cross aldol reaction between the 3 and deprotonated ketone, followed by a cyclization step, leads to the quinoline.
Conclusions
We have developed a metal-free NHC-catalyzed indirect Friedländer annulation of ketones with 2-aminobenzyl alcohol or derivatives from it to furnish functionalized quinolines in good to excellent yields and the synthesis of 2, 3-substituted quinolines has been achieved through a one-pot, two-step tandem reaction. With its multiple bond-forming ability the present tandem reaction represents an attractive option for the rapid construction of 2, 3-substituted quinolines library based on small organic molecules. The method can avoid products containing toxic metals, makes it a choice for the pharmaceutical and chemical industries.Acknowledgements
We are grateful for financial support of the Province Natural Science Foundation of Jiangsu (BK20131346).Notes and references
- X. Zhang, M. A. Campo, T. Yao and R. C. Larock, Org. Lett., 2005, 7, 763 CrossRef CAS PubMed ; and references cited therein.
- N. Kaila, K. Janz, S. DeBernardo, P. W. Bedard, R. T. Camphausen, S. Tam, D. H. H. Tsao, J. C. Keith Jr, C. Nickerson-Nutter, A. Shilling, R. Young-Sciame and Q. Wang, J. Med. Chem., 2007, 50, 21 CrossRef CAS PubMed.
- M. Krishnamurthy, K. Simon, A. M. Orendt and P. A. Beal, Angew. Chem., 2007, 119, 7174 CrossRef.
- M. Krishnamurthy, B. D. Gooch and P. A. Beal, Org. Lett., 2004, 6, 63 CrossRef CAS PubMed.
- J. B. Chaires, J. Ren, M. Henary, O. Zegrocka, G. R. Bishop and L. Strekowski, J. Am. Chem. Soc., 2003, 125, 7272 CrossRef CAS PubMed.
- L. Strekowski, M. Say, M. Henary, P. Ruiz, L. Manzel, D. E. Macfarlane and A. J. Bojarski, J. Med. Chem., 2003, 46, 1242 CrossRef CAS PubMed.
- T. L. Gildchrist, in Heterocyclic Chemistry, Pitman Publishing Ltd, London, 1st edn, 1985, p. 239 Search PubMed.
- B. R. McNaughton and B. L. Miller, Org. Lett., 2003, 5, 4257 CrossRef CAS PubMed.
- A. Arcadi, M. Chiarini, S. D. Giuseppe and F. Marinelli, Synlett, 2003, 203 CrossRef CAS PubMed.
- J. S. Yadav, B. V. S. Reddy and K. Premlatha, Synlett, 2004, 963 CrossRef CAS PubMed.
- J. Wu, L. Zhang and T.-N. Diao, Synlett, 2005, 2653 CrossRef CAS PubMed.
- B. Das, M. Krishnaiah, K. Laxminarayana and D. Nandankumar, Chem. Pharm. Bull., 2008, 56, 1049 CrossRef CAS.
- A. Shaabani, A. Rahmati and Z. Badri, Catal. Commun., 2008, 9, 13 CrossRef CAS PubMed.
- D. Garella, A. Barge, D. Upadhyaya, Z. Rodriguez, G. Palmisano and G. Cravotto, Synth. Commun., 2010, 40, 120 CrossRef CAS.
- S. Chauhan, R. Chakravarti, S. M. J. Zaidi, S. S. Aldeyab, V. Basireddy and A. Vinu, Synlett, 2010, 2597 CAS.
- A. Hasaninejad, M. Shekouhy and A. Zare, Catal. Sci. Technol., 2012, 2, 201 CAS.
- R. Martınez, D. J. Ramon and M. Yus, J. Org. Chem., 2008, 73, 9778 CrossRef PubMed.
- H. V. Mierde, P. V. D. Voort and F. Verpoort, Tetrahedron Lett., 2009, 50, 201 CrossRef PubMed.
- S. K. De and R. Gibbs, Tetrahedron Lett., 2005, 46, 1647 CrossRef CAS PubMed.
- C. S. Cho, W. X. Ren and S. C. Shim, Bull. Korean Chem. Soc., 2005, 26, 1286 CrossRef CAS.
- B. W. J. Chen, L. L. Chng, J. Yang, Y. Wei, J. Yang and J. Y. Ying, ChemCatChem, 2013, 5, 277 CrossRef CAS.
- H. V. Mierde, P. V. D. Voort and F. Verpoort, Tetrahedron Lett., 2008, 49, 6893 CrossRef PubMed.
- Y. F. Liang, X. F. Zhou, S. Y. Tang, Y. B. Huang, Y. S. Feng and H. J. Xu, RSC Adv., 2013, 3, 7739 RSC.
- J. L. Moore and T. Rovis, Top. Curr. Chem., 2009, 291, 77 CrossRef.
- E. M. Phillips, A. Chan and K. A. Scheidt, Aldrichimica Acta, 2009, 42, 55 CAS.
- V. Nair, S. Vellalath and B. P. Babu, Chem. Soc. Rev., 2008, 37, 2691 RSC.
- S. Diez-Gonzalez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612 CrossRef CAS PubMed.
- S. Wurtz and F. Glorius, Acc. Chem. Res., 2008, 41, 1523 CrossRef PubMed.
- E. A. B. Kantchev, C. J. Obrien and M. G. Organ, Angew. Chem., Int. Ed., 2007, 46, 2768 CrossRef CAS PubMed.
- E. M. Phillips, M. Riedrich and K. A. Scheidt, J. Am. Chem. Soc., 2010, 132, 13179 CrossRef CAS PubMed.
- J. M. O'Brien and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 7712 CrossRef PubMed.
- T. Boddaert, Y. Coquerel and J. Rodriguez, Chem.–Eur. J., 2011, 17, 2266 CrossRef CAS PubMed.
- R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719 CrossRef CAS.
- V. Nair, S. Vellalath and B. P. Babu, Chem. Soc. Rev., 2008, 37, 2691 RSC.
- J. L. Moore and T. Rovis, Top. Curr. Chem., 2010, 291, 77 CrossRef CAS.
- H. U. Vora and T. Rovis, J. Am. Chem. Soc., 2007, 129, 13796 CrossRef CAS PubMed.
- G. Q. Li, Y. Li, L. X. Dai and S. L. You, Org. Lett., 2007, 9, 3519 CrossRef CAS PubMed.
- J. W. Bode and S. S. Sohn, J. Am. Chem. Soc., 2007, 129, 13798 CrossRef CAS PubMed.
- K. J. Wu, G. Q. Li, Y. Li, L. X. Dai and S. L. You, Chem. Commun., 2011, 47, 493 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization of new products. See DOI: 10.1039/c4ra07858f |
| This journal is © The Royal Society of Chemistry 2014 |




