DOI:
10.1039/C4RA05500D
(Paper)
RSC Adv., 2014,
4, 52903-52910
Oxygen sensor utilizing ultraviolet irradiation assisted ZnO nanorods under low operation temperature†
Received
9th June 2014
, Accepted 2nd October 2014
First published on 2nd October 2014
Abstract
This paper presents a novel ultraviolet (UV) irradiation assisted nanostructured ZnO film for high performance oxygen sensing under a low working temperature. Nanorod ZnO structures with high exposing area are synthesized on a glass substrate with interdigital sensing electrodes via the developed two-stage sol–gel and hydrothermal processes. A UV-LED with an emission wavelength of 370 nm is then used to enhance the sensing performance of the nanostructured ZnO film. The oxygen sensor can work at a temperature of 50 °C with the assistance of UV irradiation. The response of the UV-assisted ZnO film is 4.66 times larger than the same film without UV exposure. The method developed in the present study provides a simple yet high performance method for oxygen sensing under low operation temperatures.
1. Introduction
Oxygen is one of the most important elements required to sustain life because it plays significant roles in respiration and oxidation for all living things. The monitoring of environmental oxygen content is essential in various fields including the medicine, aquaculture, farming, chemical industries and fuel combustion.1,2 The ambient oxygen concentration is also considered to be a safety factor for industrial applications.3 For example, a highly ambient oxygen level is risky for explosive, but low oxygen content can cause gas poisoning for underground engineers. The dissolved oxygen concentration in water is typically maintained to about 5–8 ppm for aquaculture applications.4 Therefore, there is an urgent demand for developing high performance and cost-efficient oxygen sensors. Oxygen concentrations in an environment can be determined with various measuring principles including the electrochemical redox detections or the solid-state sensors.2,5,6 Among these sensing methods, solid-state ceramic films, such as metal oxides fabricated with vapor deposition processes, are the most promising approach for producing commercial oxygen sensors.7 These devices have several advantages including low cost, small size, ease of integration with electronic circuit and a relatively low power consumption.
Semiconductor-based metal oxides have been used for gas sensing since 1962, when Seiyama et al. used a thin layer of zinc oxide to detect volatile organic compounds (VOC) of propane and n-butane.8 Inspired by the work, a number of metal oxide layers, such as TiO2,9–11 SnO212–14 and In2O3,15,16 were used as the sensing materials for various gases detections. However, metal oxide-based gas sensors usually rely on a high working temperature to enhance catalytic properties of the metal oxides for gas sensing. The high working temperature for this type of sensor consumes more energy for heating the sensing element and also reduces the lifetime of the sensor. In contrast, the sensing performance of these metal-oxide based sensors dramatically decreases at lower working temperatures. Therefore, a number of methods have been developed to enhance the sensing performance of the metal-oxide based gas sensors. Modifying the metal oxide layer by doping a trace amount of transient metal, such as Cr, Fe or Pd, is the most simple and straight forward way to enhance the sensing performance of these sensors.17–19 Introducing the structural defects into the material matrix to increase the carrier mobility using heterogeneous structure20–22 or producing porous structures to increase the exposed sensing area23,24 were also common approaches for the enhancement of sensing performance. For example, a metal oxide layer comprising a ZnO/SnO2 hetero-contact interface for detecting CO was reported.25 Chen et al.26 used the α-Fe2O3/SnO2 core–shell structure for ethanol detection at an operating temperature of 220°C. Recently, a Pd-doped SnO2 was reported to detect formaldehyde of the concentration as low as 50 ppb at 190 °C.27 These approaches were capable of enhancing the detection performance of the gas sensors in a simple way. However, the fabrication of heterogeneously structured metal oxides usually needs a high sintering temperature. Moreover, these gas sensors also require a relative high working temperature (higher than 150 °C) to reach the catalytic temperature.6 Nanostructured metal oxides were popularly employed in producing high performance sensors because of their excellent catalytic behaviors and high surface-to-volume ratio. The sensing performance of these gas sensors was greatly enhanced, and the working temperature was also significantly reduced. Nevertheless, this work is performed to further enhance the performance of the nanostructured ZnO metal oxide sensing layer.
Ultraviolet (UV) is a high energy beam with the photon energies from 3.0 to 12.4 eV (400–10 nm), which overlaps with the band gap of zinc oxide (around 3.4 eV).28 Therefore, UV exposure can be an efficient way to excite the hole–electron pairs in the sensing materials, such that the measured resistance of the semiconductor metal oxides is significantly reduced.29 Recently, the interaction between light irradiation and ZnO surface was investigated.30 The light-induced electrons in ZnO may attract the high electronegative molecules, such as oxygen or fluorine, and minimize the mobility of the electrons. The resistance of the sensing material increases with the increasing amount of the attracted oxygen molecules.31 In this regards, UV irradiation can enhance the sensing performance of the gas sensors without using the high working temperature to produce the electron–hole pairs. With this sensing mechanism, there are a number of researches in the recent years that have demonstrated the use of a nanostructured ZnO layer with UV radiation for gas sensing. For example, Peng et al. reported a ZnO nanorod-based formaldehyde sensor with a 120-fold sensitivity enhancement under UV exposure.32 However, the nanorod structured ZnO was grown parallel to the substrate surface, such that the exposed surface area was limited. Fan et al.33 presented the use of packed polycrystalline ZnO nanoparticles for NO2. Results showed that the ppm-level of NO2 could be detected with the assistance of UV exposure. Nevertheless, the fabrication process for packing the ZnO nanoparticles into a line trench was delicate and time-consuming.
To enhance the sensing performance under low operation temperatures, this study reports a simple and reliable method to produce out-of-plane ZnO nanorods for oxygen detection. The grass-like ZnO structure is produced via two-stage sol–gel and hydrothermal processes. The highly exposed area of the out-of-plane ZnO nanorod greatly enhances the UV exposure efficiency. In contrast, a lower dark current can be obtained due to small contact area to the substrate electrode.34 Therefore, the resistance change due to the UV exposure is greatly increased, such that oxygen detection can be achieved without applying a high working temperature. The surface morphology of ZnO nanostructures and the sensing performance produced with different ZnO producing processes are systematically inspected in the present study. The repeatability of the developed oxygen sensor utilizing UV-assisted ZnO nanorods is also experimentally measured to evaluate the performance of the oxygen sensor under a low working temperature.
2. Materials and methods
A. Sensing mechanism
Fig. 1 presents the working principle of the proposed oxygen sensing method. The high exposed area of the nanostructured ZnO sensing layer is beneficial for a highly efficient carrier induction. The UV irradiation on the ZnO nanostructures can induce excited electrons on the material surface and reduce the resistance of the sensing layer. The mechanism regarding the photoelectric response of the nanostructured ZnO have been related to the surface adsorbed species and the volume process.35 The oxygen molecules are attracted by the UV induced electrons, which form O2−(ads) because of the greater electronegativity.36 The conductivity of the ZnO is then decreased due to deceasing electron carriers in the sensing layer, resulting in an increasing measured resistivity. In contrast, the ZnO nanorods without applying UV exposure surface exhibited fewer electrons, resulting in a lower affinity for oxygen molecules adsorbing onto their surface. In general, the decreased conductivity is promotional to the adsorbed oxygen molecules on the surface of nanostructured ZnO. High performance oxygen detection can be achieved at low working temperatures with the assistance of UV irradiation.
 |
| | Fig. 1 Schematic showing the mechanism for enhancing the sensing performance of the nanostructured ZnO oxygen sensor utilizing UV irradiation. | |
B. Chip fabrication and synthesis of nanostructured ZnO
Fig. 2 presents the fabrication process of the oxygen sensing chip. A low-cost soda-lime glass was used as the substrate for developed oxygen sensor. Interdigital sensing electrodes were patterned with a standard photolithography process and metal etching process (Fig. 2A). Chromium and gold metal layers with the thicknesses of 500 Å and 2000 Å were first deposited on the glass substrate, respectively. The substrates were spin-coated with AZ4620 photoresist and soft-baked at 100 °C for 3 min. The interdigital area was patterned using a standard UV lithography process. The patterned substrate was then immersed into a developer (AZ400K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DI water = 1
DI water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). After rinsing with DI water, the substrates were immersed into gold etchant (type TFA). The substrates were then rinsed with DI water and immersed into chromium etchant (Cr-7T). After the completion of metal etching, the photoresist layer was removed with acetone to expose the metal electrodes (Fig. 2B). The nanostructured ZnO sensing layer were synthesized on a substrate with interdigital electrodes by the sol–gel process to form the ZnO seed layer, and then a hydrothermal process was employed to grow the grass-like ZnO nanorods. The reagents zinc acetate (Zn(CH3COO)2·2H2O) and hexamethylenetetramine (C6H12N4) were purchased from Panreac-Quimica (Barcelona, Spain). Ethanolamine (C2H7NO) was purchased from Sigma-Aldrich (Louis, USA). Methanol (CH4O) was obtained from Echo Chemical Co., Ltd. (Miaoli, Taiwan). All of the chemical reagents and solvents were of analytical grade. A 0.1 M precursor solution was prepared by dissolving 2 × 10−3 moles of zinc acetate in 20 ml of methanol. Once the solution was prepared, 2 × 10−3 moles of ethanolamine was added into this precursor solution. The solution was then stir-mixed and heated at 60 °C for 1 hour and stored for one day. The ZnO precursor solution was spun onto a substrate with an interdigital electrode with 1000 rpm for 20 s and then baked at 100 °C for 10 min (Fig. 2C). The spun substrate with ZnO precursor was sintered in at 200°C for 30 min then ramped to 400 °C for 0.5 hour to form the ZnO seed layer on the substrate with interdigital electrodes (Fig. 2D). After producing the seed layer, the substrate was then immersed into a mixed solution composed of 10−2 M zinc acetate and 10−2 M hexamethyltetramine for hydrothermally growing the ZnO nanorods. The hydrothermal process was maintained at 90 °C for 30 minutes to grow ZnO nanorods from the seeds (Fig. 2E). The substrate was finally cleaned with 95% alcohol followed by DI water and then dried on a hotplate (Fig. 2F).
3). After rinsing with DI water, the substrates were immersed into gold etchant (type TFA). The substrates were then rinsed with DI water and immersed into chromium etchant (Cr-7T). After the completion of metal etching, the photoresist layer was removed with acetone to expose the metal electrodes (Fig. 2B). The nanostructured ZnO sensing layer were synthesized on a substrate with interdigital electrodes by the sol–gel process to form the ZnO seed layer, and then a hydrothermal process was employed to grow the grass-like ZnO nanorods. The reagents zinc acetate (Zn(CH3COO)2·2H2O) and hexamethylenetetramine (C6H12N4) were purchased from Panreac-Quimica (Barcelona, Spain). Ethanolamine (C2H7NO) was purchased from Sigma-Aldrich (Louis, USA). Methanol (CH4O) was obtained from Echo Chemical Co., Ltd. (Miaoli, Taiwan). All of the chemical reagents and solvents were of analytical grade. A 0.1 M precursor solution was prepared by dissolving 2 × 10−3 moles of zinc acetate in 20 ml of methanol. Once the solution was prepared, 2 × 10−3 moles of ethanolamine was added into this precursor solution. The solution was then stir-mixed and heated at 60 °C for 1 hour and stored for one day. The ZnO precursor solution was spun onto a substrate with an interdigital electrode with 1000 rpm for 20 s and then baked at 100 °C for 10 min (Fig. 2C). The spun substrate with ZnO precursor was sintered in at 200°C for 30 min then ramped to 400 °C for 0.5 hour to form the ZnO seed layer on the substrate with interdigital electrodes (Fig. 2D). After producing the seed layer, the substrate was then immersed into a mixed solution composed of 10−2 M zinc acetate and 10−2 M hexamethyltetramine for hydrothermally growing the ZnO nanorods. The hydrothermal process was maintained at 90 °C for 30 minutes to grow ZnO nanorods from the seeds (Fig. 2E). The substrate was finally cleaned with 95% alcohol followed by DI water and then dried on a hotplate (Fig. 2F).
 |
| | Fig. 2 A simplified fabrication process for producing the as-developed oxygen sensor via the sol–gel and hydrothermal synthesis processes. | |
C. Oxygen detection
The sensing performance including the resistance response and time response of the produced ZnO-based oxygen sensor were characterized in a home-built chamber. A 1 W UV-LED with the major emission wavelength of 370 nm was used to enhance the sensing performance of the developed sensor. The measured surface irradiance on the sensor surface was 0.15 mW cm−2. The resistance change of the sensing layer was measured using a LCR meter (U1732C, Agilent, USA). The measured frequency for the resistance response of the oxygen sensor was set at 1 kHz. The measured resistance was acquired and recorded using a PC via an IR-USB connecting cable. For measuring the resistance response, the chamber was first pumped to 5 × 10−2 mTorr and then set to the desired oxygen concentration by injecting pure oxygen and pure nitrogen into the chamber using a mass flow controller (MFC). The sensing performance of the sensors was evaluated with a defined parameter “Response”.| |
 | (1) |
where R0 is the initial resistance of the sensor, and ΔR is the measured resistance change corresponding to the change of oxygen concentration. The sensitivity (S) was defined as the ratio of Response/Δ% O2 to further quantitatively evaluate the sensing performance of the developed sensors. The equation for the definition of sensitivity is described as follows, where Δ% O2 was the change in the oxygen concentration.| |
 | (2) |
3. Results and discussion
Fig. 3 shows the XRD patterns of the synthesized ZnO nanorod layer for oxygen detection. The diffraction peaks for 2θ at 31.77°, 34.42°, 36.25°, 47.54°, 56.6°, 62.87° and 67.96° can be attributed to the (100), (002), (101), (102), (110), (103), and (112) crystalline planes, respectively. It was found that the crystalline structure of the ZnO nanorods is wurtzite because the diffraction pattern of the produced ZnO nanorods matches with JCPDS card # 36-1451.37 The XRD result confirms the high purity of the produced ZnO nanorods. The high intensity of (002) diffraction peak illustrates that the synthesized ZnO nanorods exhibited significant tendency for growing along the c-axis. Fig. 4 shows the close-up view for the surface morphology of the synthesized ZnO nanostructures utilizing various procedures. Fig. 4A shows the sol–gel grown ZnO seed layer prior to the hydrothermal growth for the nanorods. The SEM image indicates that the ultra-fine ZnO nanospheres with a size of about 10 nm were deposited on the substrate. Fig. 4B shows the synthesized ZnO nanospheres by the sol–gel method with 0.5 M of precursor bath. A higher precursor concentration resulted in a bigger grown ZnO particle such that the size of these nanospheres was about 20 nm. It can also be noted that there were some micro-cracks observed on the surface of the as-grown ZnO layer. These cracks could be formed due to the mismatched thermal expansion coefficient between the substrate and the formed ZnO layer under the high sintering temperature. Fig. 4C shows the top view image for the surface morphology of synthesized ZnO nanorods. The SEM image showed that the nanostructured ZnO was uniformly distributed on the substrate, and the diameter of grown ZnO nanorods was about 40 nm. The extended surface area of the ZnO nanorods greatly enhanced the exposed area in comparison to the nanospheres ZnO sensing layer. Fig. 4D shows the side-view image of synthesized ZnO nanorods. Results again confirmed that the growth direction of the nanorods was perpendicular to the substrate, indicating that the ZnO was grown along the c-axis. It can also be noted that the length of ZnO nanorods was about 600 nm.
 |
| | Fig. 3 XRD diffractogram of the synthesized ZnO nanorod after the two-stage sol–gel and hydrothermal processes. Note that the peaks of Au were from the interdigital electrode. | |
 |
| | Fig. 4 SEM images showing (A) sol–gel grown nanosphere seed-layer, (B) sol–gel grown nanospheres, (C) hydrothermally grown nanorods and (D) the cross-section of the nanorod structure. | |
Fig. 5 presents the measured response after exposing the ZnO nanorod sensor into 97% oxygen with and without UV irradiation under different operation temperatures. Results showed that the response of ZnO nanorods with UV irradiation was significantly higher than the same film without UV irradiation at all operation temperatures. The measured results also showed that the UV irradiated ZnO sensing layer exhibited a lower R0 compared to the same film without UV irradiation. The results supported that the ZnO nanorods surface has more conductive carriers in the sensing layer. It is also noted that the response of the developed oxygen sensor increased with the increasing operation temperature in a certain temperature range, which is similar to other reports.27 It is also noted that the sensing response of UV-assisted ZnO oxygen sensor decreased at an operation temperature of 250 °C. This might be caused by the thermal expansion induced delamination between the nanostructured ZnO sensing layer and the glass substrate at this high temperature. Fig. 6 shows the measured response for detecting 97% oxygen using various ZnO sensing layers with and without UV irradiation at a low operation temperature of 50 °C. Results showed that the grass-like ZnO nanorod sensing layer with UV irradiation exhibited the highest response because of its large exposed surface area. The measured response for the UV irradiated ZnO nanorods sensing layer was 419%, while the same sensing layer without UV irradiation showed the response of only 74%. Results showed that the UV irradiation enhanced the sensing response by 4.66 times compared to the same sensing layer without UV irradiation. The UV irradiation was an efficient way to enhance the sensing performance of ZnO-based gas sensor. Fig. 7 presents the measured time response of various sensing layer with and without UV irradiation at the low working temperature of 50 °C. The calculated adsorption (90% response) and desorption times (63% response) for the UV-assisted nanorod ZnO were 286 and 54 s, respectively. Results showed the time response of the developed UV-assisted nanorod ZnO sensing film was considerably faster than the same sensor without UV irradiation. The changing rate for the electrical resistance of the ZnO nanorods was expected to be faster than the sensing layer without UV irradiation due to the point-junction property of the ZnO layer.38 Those point junctions in the sensing material provided the potential barriers such that the electrical resistance changed rapidly with the oxygen sensing. In addition, the ZnO nanospheres showed the slowest adsorption behavior due to the aggregation of the ZnO nanoparticles, resulting in a longer diffusion path for oxygen molecules. The repeating tests showed that the developed sensor had good repeatability and stability for oxygen detection. Fig. 8 shows three repeating measurement for detecting 97% oxygen to evaluate the repeatability for the developed sensor with UV irradiation. The calculated variation for these three measurements was only 3.3%, indicating the efficient reproducibility of the ZnO-based oxygen sensor.
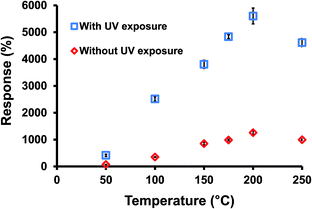 |
| | Fig. 5 The measured sensing responses of ZnO nanorods under various operation temperatures. | |
 |
| | Fig. 6 The measured sensing responses of various ZnO sensing layers with and without UV irradiation at 50 °C. | |
 |
| | Fig. 7 The measured time response of various ZnO sensing layers with and without UV irradiation at 50 °C. | |
 |
| | Fig. 8 Three repeating tests for detecting 97% of oxygen using the developed oxygen sensor with UV irradiation at 50 °C. | |
Fig. 9 shows the measured sensing performance of the developed UV-assisted ZnO nanorods for detecting oxygen at a low working temperature of 50 °C. Fig. 9A shows the continuous measurement for the step response for detecting oxygen at different pressures. Fig. 9B presents the relationship between the measured responses and the oxygen pressures. Note that the measurement was performed in a high vacuum chamber under a precise pressure control. Results showed that a nice linearity (R2 = 0.9952) from 5 to 1000 mTorr was obtained, confirming the good sensing performance of the developed sensor. Results also indicated that the limit of detection (LOD) for the oxygen sensor was as low as 5 mTorr. The calculated sensitivity of the sensor was 0.17 (%/mTorr O2). The LOD and sensitivity of the developed sensor were 64.4 and 3.1 times greater than the reported cobalt-doped ZnO nanofibres, respectively.39 Fig. 10 presents the measured sensing performance of the developed sensor for detecting oxygen concentration at 50 °C under 1 atm. Fig. 10A shows the measured step response for detecting oxygen at different ratios. Fig. 10B presents the relationship between the measured responses under various oxygen ratios. The chamber was purged to 1 atm with the sample gas composed of different oxygen ratios (mixed with pure nitrogen) using a precision MFC. Results indicated that nice linearity (R2 = 0.9337) from 10% to 97% of oxygen ratio was obtained. The calculated sensitivity for the developed oxygen sensor was 1.83 (%/% O2) under 1 atm of measuring condition. The sensitivity of the developed sensor was 3.5 times higher than the SnO2-gated AlGaN/GaN transistor under 1 atm.40 In addition, the developed sensor also exhibited the sensitivity about 4.5 times higher than that of the multi-walled carbon nanotubes based oxygen sensor under the same condition.41 The experimental results confirmed the wide detection range and good sensing performance for the developed oxygen sensor assisted with UV irradiation. The developed oxygen sensor utilizing UV-assisted ZnO nanorods showed its potential to be a high performance oxygen sensor, which can be operated at a low working temperature.
 |
| | Fig. 9 (A) The measured step response for detecting oxygen at different pressures. (B) The relationship between the measured responses and the oxygen pressures. (With UV irradiation at 50 °C.) | |
 |
| | Fig. 10 (A) The measured step response for detecting oxygen at different concentrations. (B) The relationship between the measured responses and the oxygen concentrations. (With UV irradiation at 50 °C.) | |
4. Conclusions
A high performance oxygen sensor utilizing UV-assisted ZnO nanorods under a low operation temperature has been developed. A simple two-stage sol–gel and hydrothermal processes was used to synthesize the ZnO nanorods for oxygen detection. The grass-like nanostructured ZnO sensing layer exhibited a high expose area to the oxygen sample and for UV irradiation. A low-cost UV LED with the wavelength of 370 nm was used to efficiently induce electron carriers in the nanostructured ZnO sensing layer for the enhancement of sensing performance. The measured resistance of the ZnO sensing layer significantly reduced with UV irradiation, indicating an increase for conductive carriers in the ZnO and an enhancement without using a high working temperature. Results indicated that the developed sensor has a low limit of detection as low as 5 mTorr for detecting oxygen with a short time response of less than 5 min. The calculated variation for three repeating tests for the sensor was around 3.3%, indicating the nice reproducibility for the oxygen sensor. The calculated sensitivities are 0.17 (%/mTorr O2) and 1.83 (%/% O2) for detecting oxygen of different pressures and concentrations, respectively. Therefore, the developed oxygen sensor showed its potential to be a high performance oxygen sensor under a low working temperature.
References
- Q. S. Ding, D. K. Ma, D. L. Li, Y. G. Wei and N. N. Wen, A Wireless Control Node for Dissolved Oxygen in Aquaculture, Sens. Lett., 2013, 11, 1069–1073 CrossRef CAS.
- W. C. Maskell, Inorganic Solid-State Chemically Sensitive Devices – Electrochemical Oxygen Gas Sensors, J. Phys. E: Sci. Instrum., 1987, 20, 1156–1168 CrossRef CAS.
- M. Ryden, E. Cleverstam, A. Lyngfelt and T. Mattisson, Waste products from the steel industry with NiO as additive as oxygen carrier for chemical-looping combustion, Int. J. Greenhouse Gas Control, 2009, 3, 693–703 CrossRef CAS.
- B. B. Jana and D. Sarkar, Water quality in aquaculture – Impact and management: A review, Indian J. Anim. Sci., 2005, 75, 1354–1361 CAS.
- H. Minaee, S. H. Mousavi, H. Haratizadeh and P. W. de Oliveira, Oxygen sensing properties of zinc oxide nanowires, nanorods, and nanoflowers: The effect of morphology and temperature, Thin Solid Films, 2013, 545, 8–12 CrossRef CAS.
- G. Korotcenkov, Metal oxides for solid-state gas sensors: What determines our choice?, J. Mater. Sci. Eng. B, 2007, 139, 1–23 CrossRef CAS.
- P. Mitra, A. P. Chatterjee and H. S. Maiti, ZnO thin film sensor, Mater. Lett., 1998, 35, 33–38 CrossRef CAS.
- T. Seiyama, A. Kato, K. Fujiishi and M. Nagatani, A New Detector for Gaseous Components Using Semiconductive Thin Films, Anal. Chem., 1962, 34, 1502–1503 CrossRef CAS.
- O. K. Varghese, D. Gong, M. Paulose, K. G. Ong and C. A. Grimes, Hydrogen sensing using titania nanotubes, Sens. Actuators, B, 2003, 93, 338–344 CrossRef CAS.
- P. Hu, G. Du, W. Zhou, J. Cui, J. Lin and H. Liu, et al., Enhancement of ethanol vapor sensing of TiO2 nanobelts by surface engineering, ACS Appl. Mater. Interfaces, 2010, 2, 3263–3269 CAS.
- L. Francioso, A. Taurino, A. Forleo and P. Siciliano, TiO2 nanowires array fabrication and gas sensing properties, Sens. Actuators, B, 2008, 130, 70–76 CrossRef CAS.
- D. Wang, X. Chu and M. Gong, Gas-sensing properties of sensors based on single-crystalline SnO2 nanorods prepared by a simple molten-salt method, Sens. Actuators, B, 2006, 117, 183–187 CrossRef CAS.
- J.-K. Choi, I.-S. Hwang, S.-J. Kim, J.-S. Park, S.-S. Park and U. Jeong, et al., Design of selective gas sensors using electrospun Pd-doped SnO2 hollow nanofibers, Sens. Actuators, B, 2010, 150, 191–199 CrossRef CAS.
- T. Le Viet, N. D. Hoa, D. T. T. Le, V. Do Thanh, P. D. Tam and A.-T. Le, et al., On-chip fabrication of SnO2-nanowire gas sensor: The effect of growth time on sensor performance, Sens. Actuators, B, 2010, 146, 361–367 CrossRef.
- X. Lu and L. Yin, Porous Indium oxide nanorods: Synthesis, characterization and gas sensing properties, J. Mater. Sci. Technol., 2011, 27, 680–684 CAS.
- W. Zheng, X. Lu, W. Wang, Z. Li, H. Zhang and Z. Wang, et al., Assembly of Pt nanoparticles on electrospun In2O3 nanofibers for H2S detection, J. Colloid Interface Sci., 2009, 338, 366 CrossRef CAS PubMed.
- R. Sharma, M. Bhatnagar and G. Sharma, Mechanism of highly sensitive and fast response Cr doped TiO2 oxygen gas sensor, Sens. Actuators, B, 1997, 45, 209–215 CrossRef CAS.
- A. Kolmakov, D. Klenov, Y. Lilach, S. Stemmer and M. Moskovits, Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles, Nano Lett., 2005, 5, 667–673 CrossRef CAS PubMed.
- H.-T. Wang, B. Kang, F. Ren, L. Tien, P. Sadik and D. Norton, et al., Hydrogen-selective sensing
at room temperature with ZnO nanorods, Appl. Phys. Lett., 2005, 86, 243503–243503-3 CrossRef.
- X. Xue, L. Xing, Y. Chen, S. Shi, Y. Wang and T. Wang, Synthesis and H2S Sensing Properties of CuO–SnO2 Core–Shell PN-Junction Nanorods, J. Phys. Chem. C, 2008, 112, 12157–12160 CAS.
- S. Si, C. Li, X. Wang, Q. Peng and Y. Li, Fe2O3/ZnO core–shell nanorods for gas sensors, Sens. Actuators, B, 2006, 119, 52–56 CrossRef CAS.
- N. Van Hieu, H.-R. Kim, B.-K. Ju and J.-H. Lee, Enhanced performance of SnO2 nanowires ethanol sensor by functionalizing with La2O3, Sens. Actuators, B, 2008, 133, 228–234 CrossRef.
- Z. Guo, M. Li and J. Liu, Highly porous CdO nanowires: preparation based on hydroxy-and carbonate-containing cadmium compound precursor nanowires, gas sensing and optical properties, Nanotechnology, 2008, 19, 245611 CrossRef PubMed.
- G. Wang, J. Park, M. Park and X. L. Gou, Synthesis and high gas sensitivity of tin oxide nanotubes, Sens. Actuators, B, 2008, 131, 313–317 CrossRef CAS.
- H. Yu and M. Choi, Electrical and CO gas sensing properties of ZnO–SnO2 composites, Sens. Actuators, B, 1998, 52, 251–256 CrossRef.
- Y.-J. Chen, C.-L. Zhu, L.-J. Wang, P. Gao, M.-S. Cao and X.-L. Shi, Synthesis and enhanced ethanol sensing characteristics of α-Fe2O3/SnO2 core–shell nanorods, Nanotechnology, 2009, 20, 045502 CrossRef PubMed.
- S. Q. Tian, X. H. Ding, D. W. Zeng, J. J. Wu, S. P. Zhang and C. S. Xie, A low temperature gas sensor based on Pd-functionalized mesoporous SnO2 fibers for detecting trace formaldehyde, RSC Adv., 2013, 3, 11823–11831 RSC.
- A. Janotti and C. G. Van de Walle, Fundamentals of zinc oxide as a semiconductor, Rep. Prog. Phys., 2009, 72, 126501 CrossRef.
- D. Gedamu, I. Paulowicz, S. Kaps, O. Lupan, S. Wille and G. Haidarschin, et al., Rapid Fabrication Technique for Interpenetrated ZnO Nanotetrapod Networks for Fast UV Sensors, Adv. Mater., 2014, 26, 1541–1550 CrossRef CAS PubMed.
- R. Gurwitz, R. Cohen and I. Shalish, Interaction of light with the ZnO surface: Photon induced oxygen “breathing,” oxygen vacancies, persistent photoconductivity, and persistent photovoltage, J. Appl. Phys., 2014, 115, 033701 CrossRef.
- N. Sakai, Y. Ebina, K. Takada and T. Sasaki, Photocurrent generation from semiconducting manganese oxide nanosheets in response to visible light, J. Phys. Chem. B, 2005, 109, 9651–9655 CrossRef CAS PubMed.
- L. Peng, Q. Zhao, D. Wang, J. Zhai, P. Wang and S. Pang, et al., Ultraviolet-assisted gas sensing: a potential formaldehyde detection approach at room temperature based on zinc oxide nanorods, Sens. Actuators, B, 2009, 136, 80–85 CrossRef CAS.
- S.-W. Fan, A. K. Srivastava and V. P. Dravid, Nanopatterned polycrystalline ZnO for room temperature gas sensing, Sens. Actuators, B, 2010, 144, 159–163 CrossRef CAS.
- L. Ji, S. Peng, Y.-K. Su, S.-J. Young, C. Wu and W. Cheng, Ultraviolet photodetectors based on selectively grown ZnO nanorod arrays, Appl. Phys. Lett., 2009, 94, 203106 CrossRef.
- O. Lupan, L. Chow and G. Y. Chai, A single ZnO tetrapod-based sensor, Sens. Actuators, B, 2009, 141, 511–517 CrossRef CAS.
- B. M. Rajbongshi and S. K. Samdarshi, ZnO and Co–ZnO nanorods – Complementary role of oxygen vacancy in photocatalytic activity of under UV and visible radiation flux, Mater. Sci. Eng., B, 2014, 182, 21–28 CrossRef CAS.
- O. Lupan, T. Pauporte, L. Chow, B. Viana, F. Pelle and L. K. Ono, et al., Effects of annealing on properties of ZnO thin films prepared by electrochemical deposition in chloride medium, Appl. Surf. Sci., 2010, 256, 1895–1907 CrossRef CAS.
- H.-U. Lee, K. Ahn, S.-J. Lee, J.-P. Kim, H.-G. Kim and S.-Y. Jeong, et al., ZnO nanobarbed fibers: Fabrication, sensing NO2 gas, and their sensing mechanism, Appl. Phys. Lett., 2011, 98, 193114 CrossRef.
- M. Yang, T. Xie, L. Peng, Y. Zhao and D. Wang, Fabrication and photoelectric oxygen sensing characteristics of electrospun Co doped ZnO nanofibres, Appl. Phys. A: Mater. Sci. Process., 2007, 89, 427–430 CrossRef CAS.
- S. Hung, C.-J. Chung, C. C. Chen, C. Lo, F. Ren and S. Pearton, et al., SnO2-gated AlGaN/GaN high electron mobility transistors based oxygen sensors, J. Vac. Sci. Technol., B: Nanotechnol. Microelectron.: Mater., Process., Meas., Phenom., 2012, 30, 041214 CrossRef.
- C. Cava, R. Salvatierra, D. Alves, A. Ferlauto, A. Zarbin and L. Roman, Self-assembled films of multi-wall carbon nanotubes used in gas sensors to increase the sensitivity limit for oxygen detection, Carbon, 2012, 50, 1953–1958 CrossRef CAS.
Footnote |
| † The preliminary results of the present study have been reported at the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems (IEEE NEMS 2013), Suzhou, China, Apr. 7–10, 2013. |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DI water = 1
DI water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). After rinsing with DI water, the substrates were immersed into gold etchant (type TFA). The substrates were then rinsed with DI water and immersed into chromium etchant (Cr-7T). After the completion of metal etching, the photoresist layer was removed with acetone to expose the metal electrodes (Fig. 2B). The nanostructured ZnO sensing layer were synthesized on a substrate with interdigital electrodes by the sol–gel process to form the ZnO seed layer, and then a hydrothermal process was employed to grow the grass-like ZnO nanorods. The reagents zinc acetate (Zn(CH3COO)2·2H2O) and hexamethylenetetramine (C6H12N4) were purchased from Panreac-Quimica (Barcelona, Spain). Ethanolamine (C2H7NO) was purchased from Sigma-Aldrich (Louis, USA). Methanol (CH4O) was obtained from Echo Chemical Co., Ltd. (Miaoli, Taiwan). All of the chemical reagents and solvents were of analytical grade. A 0.1 M precursor solution was prepared by dissolving 2 × 10−3 moles of zinc acetate in 20 ml of methanol. Once the solution was prepared, 2 × 10−3 moles of ethanolamine was added into this precursor solution. The solution was then stir-mixed and heated at 60 °C for 1 hour and stored for one day. The ZnO precursor solution was spun onto a substrate with an interdigital electrode with 1000 rpm for 20 s and then baked at 100 °C for 10 min (Fig. 2C). The spun substrate with ZnO precursor was sintered in at 200°C for 30 min then ramped to 400 °C for 0.5 hour to form the ZnO seed layer on the substrate with interdigital electrodes (Fig. 2D). After producing the seed layer, the substrate was then immersed into a mixed solution composed of 10−2 M zinc acetate and 10−2 M hexamethyltetramine for hydrothermally growing the ZnO nanorods. The hydrothermal process was maintained at 90 °C for 30 minutes to grow ZnO nanorods from the seeds (Fig. 2E). The substrate was finally cleaned with 95% alcohol followed by DI water and then dried on a hotplate (Fig. 2F).
3). After rinsing with DI water, the substrates were immersed into gold etchant (type TFA). The substrates were then rinsed with DI water and immersed into chromium etchant (Cr-7T). After the completion of metal etching, the photoresist layer was removed with acetone to expose the metal electrodes (Fig. 2B). The nanostructured ZnO sensing layer were synthesized on a substrate with interdigital electrodes by the sol–gel process to form the ZnO seed layer, and then a hydrothermal process was employed to grow the grass-like ZnO nanorods. The reagents zinc acetate (Zn(CH3COO)2·2H2O) and hexamethylenetetramine (C6H12N4) were purchased from Panreac-Quimica (Barcelona, Spain). Ethanolamine (C2H7NO) was purchased from Sigma-Aldrich (Louis, USA). Methanol (CH4O) was obtained from Echo Chemical Co., Ltd. (Miaoli, Taiwan). All of the chemical reagents and solvents were of analytical grade. A 0.1 M precursor solution was prepared by dissolving 2 × 10−3 moles of zinc acetate in 20 ml of methanol. Once the solution was prepared, 2 × 10−3 moles of ethanolamine was added into this precursor solution. The solution was then stir-mixed and heated at 60 °C for 1 hour and stored for one day. The ZnO precursor solution was spun onto a substrate with an interdigital electrode with 1000 rpm for 20 s and then baked at 100 °C for 10 min (Fig. 2C). The spun substrate with ZnO precursor was sintered in at 200°C for 30 min then ramped to 400 °C for 0.5 hour to form the ZnO seed layer on the substrate with interdigital electrodes (Fig. 2D). After producing the seed layer, the substrate was then immersed into a mixed solution composed of 10−2 M zinc acetate and 10−2 M hexamethyltetramine for hydrothermally growing the ZnO nanorods. The hydrothermal process was maintained at 90 °C for 30 minutes to grow ZnO nanorods from the seeds (Fig. 2E). The substrate was finally cleaned with 95% alcohol followed by DI water and then dried on a hotplate (Fig. 2F).











