Controlled fragrance release from galactose-based pro-fragrances†
Abstract
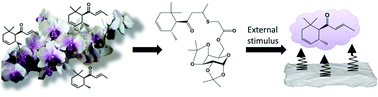
The volatile nature of olfactory compounds has led to the development of pro-fragrances, which slowly release the active fragrance molecules upon cleavage of a chemical bond to a substrate. Based on the hypothesis that monosaccharide motifs could serve to effectively anchor pro-fragrances on cotton, which is an important requirement for use in laundry products, we investigated new galactose-based pro-fragrances. A retro 1,4-Michael-type reaction was employed as the release mechanism. Thus, δ-damascone was reacted in a 1,4-addition with mercaptoacetic acid, and the product was coupled with 1,2:3,4-di-O-isopropylidene-α-D-galactopyranose. To explore the influence of the molecules' polarity on the deposition and release kinetics, both the isopropylidene-protected hydrophobic as well as the deprotected hydrophilic pro-fragrance were studied. The fragrance release was investigated in aqueous solution by 1H-NMR spectroscopy as a function of pH; the data show that both pro-fragrances are stable under acidic conditions, but release the δ-damascone under basic conditions. The release kinetics are well described by a first-order process, and observed to be much faster in the case of the isopropylidene-protected hydrophobic pro-fragrance. The fragrance release from washed and dried cotton tissue was investigated via dynamic headspace analysis followed by gas chromatography. The data show that the deposition from solution is much better for the hydrophobic pro-fragrance, that the δ-damascone is slowly released in both cases, and that the amount of δ-damascone that can be released is increased by over two orders of magnitude higher than in the case of tissue washed with the neat fragrance under identical conditions.

 Please wait while we load your content...
Please wait while we load your content...