Extraction mechanism and γ-radiation effect on the removal of Eu3+ by a novel BTPhen/[Cnmim][NTf2] system in the presence of nitric acid†
Hanyang Zhou‡
,
Yinyong Ao‡,
Jie Yuan,
Jing Peng*,
Jiuqiang Li and
Maolin Zhai*
Beijing National Laboratory for Molecular Sciences (BNLMS), Radiochemistry and Radiation Chemistry Key Laboratory of Fundamental Science, Department of Applied Chemistry, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: jpeng@pku.edu.cn; mlzhai@pku.edu.cn; Fax: +86-10-62753794; Tel: +86-10-62757193
First published on 16th September 2014
Abstract
The extraction mechanism of 2,9-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[1,2,4]triazin-3-yl)-1,10-phenanthroline (BTPhen) in combination with 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imides ionic liquids ([Cnmim][NTf2], n = 2, 4, 8) in the presence of nitric acid is studied by using Eu3+, which has similar properties to trivalent actinides. The dominant complex compound forming between BTPhen and Eu changes from [Eu(BTPhen)2(NO3)]2+ to [Eu(BTPhen)(NO3)3] gradually with the increase of [HNO3] or the length of the alkyl chain in the [Cnmim]+, indicating that the main extraction mechanism of BTPhen/[Cnmim][NTf2] varies from a cation exchange mechanism to a neutral species mechanism. The irradiation of BTPhen in solid state has no effect on the extraction of Eu3+. The abnormally enhanced removal of Eu3+ was observed in irradiated BTPhen/[C2mim][NTf2] system with 0.1 M nitric acid, and it was attributed to the formation of precipitates between Eu3+ and trace radiolytic products of [C2mim][NTf2], which could be recovered by water washing before extraction. The extraction of Eu3+ was slightly changed for the irradiated BTPhen/[C2mim][NTf2] system with 1 M nitric acid, demonstrating good radiation stability of this novel extraction system.
1. Introduction
During the reprocessing of spent nuclear fuel (SNF), the PUREX process has been widely used to separate U and Pu, but a few minor actinides (MA, mainly Am and Cm), which are responsible for high-level and long-term radiotoxicity of SNF, still exist in the outflow water solution of the PUREX process.1,2 It is necessary to remove them before further safe disposal of SNF. MA transmutation by fast neutrons, leading to residues with less radiotoxicity and shorter half-life nuclides, has been anticipated to be an effective method. However, prior to all processing it still requires another necessary step to separate them from lanthanides (Ln) which greatly exist in SNF and have higher neutron capture section than MA.3–5 However, the separation of MA–Ln is a huge challenge due to their similar chemical properties.In the past decades, tridentate 2,6-bis(1,2,4-triazine-3-yl)pyridine (BTP, Fig. 1(a)) and quadridentate 6,6′-bis(1,2,4-triazine-3-yl)-2,2′-bipyridine (BTBP, Fig. 1(b)), two main series of N-donor heteropolycyclic ligands have been widely investigated and proved to be effective in the MA–Ln separation.5–8 Some research suggested that their high selectivity is due to their relatively softer N-donor atoms and unusual orbital interactions.5,8 Moreover, compared with previously reported BTBPs, structurally modified BTBP ligand, 2,9-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo[1,2,4]triazin-3-yl)-1,10-phenanthroline (BTPhen, Fig. 1(c)), which has been recently synthesized, is reported to have higher separation factor and faster extraction kinetic in traditional extraction process for Am–Eu partition.9
Studies show that the extraction capacity of BTBPs reduces significantly under high doses γ-radiation, even though BTBP is consisted of relatively stable CyMe4-substituted ligands,10 and diluent plays a pivotal role in radiolysis of BTBPs.11 Some active species such as free radicals could be easily produced in the volatile organic diluents (e.g. 1-octanol) under radiation. H-abstraction reaction on ligand molecules induced by free radicals could lead to some subsequent radiolysis of BTBPs.11 As a consequence, the extractants would lose their extractability and selectivity. In order to apply BTPhen to the separation of MA–Ln in practical system, the evaluation of radiation stability of BTPhen is necessary, and more stable extraction system is required.
Room temperature ionic liquids (RTILs) have been considered as alternative medias to replace traditional organic diluents in the reprocessing of SNF,12,13 because of a number of unique properties such as non-volatility, good solubility, along with chemical stability, etc. in them.14,15 Among these properties, the environmental protection and the potential in reducing the critical accident are the most admirable features concerning a safety media used in the reprocessing of SNF.16–18 In addition, RTILs combining with other extractants, which show excellent extraction performance, have been studied in the separation of some radionuclides (e.g. Sr, Cs, An).19–21 However, up to now there has been no study on BTPhen/ionic liquid extraction system. Herein, we expect to design a new extraction system in which BTPhen as extractant and [Cnmim][NTf2] (Fig. 1(d)) as diluent. Since Am is known for its radiotoxicity and the difficulty in handling, while Eu3+ has similar properties with trivalent actinides, Eu3+ has been widely used on behalf of the trivalent Ln and An in the spent nuclear fuel.22–24 Therefore, Eu3+ is chosen to evaluate the extractability and the γ-radiation stability of BTPhen/[Cnmim][NTf2] system in this work.
2. Materials and methods
2.1. Materials
[Cnmim][NTf2] (n = 2, 4, 8 stands for ethyl-, butyl-, octyl-, respectively; with a purity >99%) were purchased from Lanzhou Greenchem ILs, LICP, CAS, China (Lanzhou, China). No impurities were detected using 1H NMR spectrometry, and the residual water amounts less than 400 ppm in the [Cnmim][NTf2] were determined by Karl-Fischer titration before irradiation. These compounds were used as received prior to irradiation. BTPhen was synthesized and purified according to the literature with a satisfactory purity (>95%).9 All other solvents were analytical-grade reagent and used without further purification.2.2. Extraction of Eu3+
The aqueous phase (1.0 mL) with various nitric acid concentrations and 1 mM Eu(NO3)3 was mixed with the organic phase (0.5 mL) containing 10 mM BTPhen. Then the mixtures were oscillated in a constant temperature incubator shaker at 323 ± 1 K with a rotating speed at 200 rpm for a certain period. The obtained mixtures were centrifuged for phase separation and the aqueous phase was analysed by Prodigy high dispersion inductively coupled plasma atomic emission spectrometer (ICP-AES) (Teledyne Leeman 85 Labs, USA) to determine the concentration of Eu3+. The distribution ratio (DEu) and the extraction efficiency (EEu) are calculated from eqn (1) and (2), respectively. The “[Eu]” represents the concentration of Eu3+, and the subscript “org/aq” is short for organic phase/aqueous phase, while the “i/f” represents the initial/final concentration before/after extraction.
 | (1) |
 | (2) |
2.3. Irradiation
Irradiation of solid BTPhen, [C2mim][NTf2], BTPhen/[C2mim][NTf2] and BTPhen/1-octanol solution was carried out separately at room temperature in air using a 60Co source with a dose rate of ca. 300 Gy min−1 (Institute of Applied Chemistry of Peking University). Plastic tubes were chosen as the container during irradiation to avoid the reaction between container and acidic radiolytic products.25 The absorbed dose was measured with Fricke dosimeter.2.4. Characterization
NMR experiments were recorded on a Bruker AV-500 instrument. (1) The determination of the concentration of ionic liquid in water: the solubility equilibrium of [C2mim]+ in water was achieved by contacting 0.5 mL [C2mim][NTf2] with 3 mL deuterium oxide (D2O) containing 1 mM Eu3+ and different concentrations of HNO3 for 30 seconds. The aqueous phase was partitioned and then mixed with equal volume of sodium acetate–D2O solution at 50 mM. The mixture was analysed by 1H NMR and the concentration of [C2mim]+ was calculated from eqn (3), where the “[C2mim+]” and “[CH3COO−]” are the concentrations of [C2mim]+ and CH3COO−, respectively. The “A[C2mim]+” and “ACH3COO−” are the integrals of the methyl in the [C2mim]+ and CH3COO−, respectively.
 | (3) |
(2) Solid BTPhen samples irradiated at different doses were dissolved into deuterated chloroform (CDCl3) separately and the chemical shift scale was calibrated with tetramethylsilane at 0 ppm for 1H NMR. (3) The determination of the water soluble radiolytic products of [C2mim][NTf2]: the separation of water-soluble radiolytic products from [C2mim][NTf2] was conducted by contacting 0.5 mL irradiated sample with 0.5 mL D2O for about 10 min in a vibrating mixer, followed by centrifuging to ensure that the phases were fully mixed and separated. The aqueous phase obtained by washing the irradiated sample was analysed by 19F NMR and the chemical shift scale was calibrated with C6F6 (−162.73 ppm according to the ref. 17).
Micro Fourier transform Infrared spectroscopies (Micro-FTIR) of solid BTPhen at different doses were recorded on a Thermo Scientific Micro Fourier transform infrared spectrometry.
X-ray photoelectron spectroscopy (XPS) analysis was performed with an AXIS-Ultra instrument from Kratos Analytical using monochromatic Al Kα radiation and low energy electron flooding for charge compensation. The binding energies (BE) were calibrated using C1s hydrocarbon peak at 284.80 eV.
3. Results and discussion
3.1. Extraction of Eu3+ in BTPhen/[C2mim][NTf2] system
The extraction kinetics of BTPhen/[C2mim][NTf2] system was first investigated at 0.1 M HNO3. The EEu increases rapidly and reaches a flat with a value exceeding 95% after an hour (Fig. S1†). The result indicates that the extraction equilibrium at 0.1 M HNO3 could be achieved in an hour and the Eu3+ could be almost completely extracted into organic phase after two hours. In order to study the effect of nitric acid concentration and the chemical structures of [Cnmim][NTf2] on the extraction property of the extraction systems, [Cnmim][NTf2] with different alkyl lengths in the cations were selected as the diluents. Extraction experiment was carried out at different initial nitric acid concentrations (abbreviated as [HNO3]) ranging from 0.01 M to 3 M. For purpose of comparison, two hours oscillation time was chosen for all extraction samples.As shown in Fig. 2 (data refer to Table S1†), the DEu of BTPhen/[C2mim][NTf2] system declines from 52 (0.01 M HNO3) to the minimum of 0.33 (2 M HNO3), and then slightly rises to 0.40 (3 M HNO3) with the [HNO3] increasing. The variations of DEu with the [HNO3] in [C4mim][NTf2] and [C8mim][NTf2] are similar to those in [C2mim][NTf2]. This phenomenon is also observed in extraction systems applying RTILs for other radionuclides (e.g. actinides, Sr, Cs).21,26,27 However, when traditional solvents in which better extraction results are usually obtained at high acidities are employed, this phenomenon is not common.28 That the variations of DEu of BTPhen/[Cnmim][NTf2] systems with the [HNO3] increase indicates a tendency which is similar to the “boomerang curves” previously described by Billard et al.,29 who proposed an extraction model that unified three possible basic mechanisms: cation exchange, anion exchange, and neutral complex extraction. These mechanisms are normally synergic during the extraction, whereas the dominant one depends on conditions. The boomerang curves reveal an interesting phenomenon that an extraction system employing RTILs perform well at neutral or low acidity. The extractability declines to a bottom level with the acidity increasing and then slowly rises again in a region of rather high acidity.
In order to reveal the extraction mechanism of BTPhen/[Cnmim][NTf2] system, the relationship of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEu against log[BTPhen] in [C2mim][NTf2] is measured with the previous method30 and shown in Fig. 3. The fitted slope value is reduced from 2.0 (0.1 M HNO3) to 1.0 (3 M HNO3). This result indicates the molar ratio between BTPhen and Eu of complexes changes from 2
DEu against log[BTPhen] in [C2mim][NTf2] is measured with the previous method30 and shown in Fig. 3. The fitted slope value is reduced from 2.0 (0.1 M HNO3) to 1.0 (3 M HNO3). This result indicates the molar ratio between BTPhen and Eu of complexes changes from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gradually with the [HNO3] increasing. Two kinds of complexes formed between BTPhen and Eu3+, namely [Eu(BTPhen)2(NO3)]2+ (2
1 gradually with the [HNO3] increasing. Two kinds of complexes formed between BTPhen and Eu3+, namely [Eu(BTPhen)2(NO3)]2+ (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex) and [Eu(BTPhen)(NO3)3] (1
1 complex) and [Eu(BTPhen)(NO3)3] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex), have been reported to be formed in traditional diluents based on previous report.24 Consequently, the complexes between BTPhen and Eu3+ formed in [C2mim][NTf2] are in accordance to the previous report.30 Considering the difference in the chemical structures of two complexes, the cation exchange mechanism for [Eu(BTPhen)2(NO3)]2+ and the neutral complex mechanism for [Eu(BTPhen) (NO3)3] in the presence of [Cnmim][NTf2] are proposed as eqn (4) and (5), respectively. In the equations, overlines represent that the covered species are in the RTILs phase, while the uncovered species are in the aqueous phase. In addition, Eu3+ cannot be extracted by pure [Cnmim][NTf2] in the presence of nitric acid, and thus anion exchange mechanism does not exist in this situation without BTPhen.
1 complex), have been reported to be formed in traditional diluents based on previous report.24 Consequently, the complexes between BTPhen and Eu3+ formed in [C2mim][NTf2] are in accordance to the previous report.30 Considering the difference in the chemical structures of two complexes, the cation exchange mechanism for [Eu(BTPhen)2(NO3)]2+ and the neutral complex mechanism for [Eu(BTPhen) (NO3)3] in the presence of [Cnmim][NTf2] are proposed as eqn (4) and (5), respectively. In the equations, overlines represent that the covered species are in the RTILs phase, while the uncovered species are in the aqueous phase. In addition, Eu3+ cannot be extracted by pure [Cnmim][NTf2] in the presence of nitric acid, and thus anion exchange mechanism does not exist in this situation without BTPhen.
 | (4) |
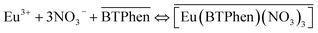 | (5) |
 | ||
Fig. 3 Variations in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEu as a function of log[BTPhen] in [C2mim][NTf2]. Initial nitric acid concentration: 0.1 M (a), 1 M (b), 3 M (c). DEu as a function of log[BTPhen] in [C2mim][NTf2]. Initial nitric acid concentration: 0.1 M (a), 1 M (b), 3 M (c). | ||
Although both of the cation exchange and neutral complex extraction mechanisms are present during the extraction, each contribution depends on the extraction conditions, especially the [HNO3]. According to the slope values displayed in Fig. 3, the dominant mechanism varies from cation exchange mechanism to neutral complex mechanism with the [HNO3] increasing. The proportion of cation exchange mechanism is restrained by the nitric acid, while the proportion of neutral complex mechanism is enhanced simultaneously. The influence of [HNO3] on the water-solubility of [C2mim]+ is evaluated by 1H NMR. The solubility equilibrium between [C2mim][NTf2] and aqueous phase is established rapidly through vibrating mixer. As shown in the Fig. 4, the solubility of [C2mim]+ evidently increases with the [HNO3] increasing which is possibly due to the cation exchange between [C2mim]+ and H+. These results indicate that the solubility equilibrium between [C2mim]+ and aqueous phase will be established firstly during the extraction, and the concentration of [C2mim]+ is positively related to the [HNO3]. According to eqn (4), higher concentration of [C2mim]+ in aqueous phase makes the cation exchange between [Eu(BTPhen)2(NO3)]2+ and [C2mim]+ less favourable. Therefore, the contribution of the cation exchange mechanism to the extraction of Eu3+ is restrained significantly with the [HNO3] increasing. Although the contribution of the neutral complex mechanism to the extraction of Eu3+ would be improved with the concentration of NO3− increasing, the effect is not significant until the [HNO3] is great enough, which is similar to that in traditional solvents.9 As a result, the DEu decreases obviously to a minimum and then slightly rises again (Fig. 2).
 | ||
| Fig. 4 The water-solubility of [C2mim]+ at different acidities. Inset: 1H NMR spectrum of the mixture solution of [C2mim]+ and sodium acetate. | ||
Moreover, the influence of the chemical structure of [Cnmim][NTf2] on the Eu3+ partitioning is also shown in Fig. 2. At controlling nitric acid concentrations, especially the low concentrations, the DEu decreases with the length of alkyl chain of [Cnmim]+ changing from 2 to 8. Fig. 5 displays the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEu − log[BTPhen] curves in three kinds of [Cnmim][NTf2]. The slope value gradually decreases with the length of alkyl chain increasing, indicating the restraining of the cation exchange mechanism. Thus, longer alkyl chain is less favourable to the cation exchange, due to higher hydrophobicity of the corresponding [Cnmim]+. Furthermore, longer alkyl chain of [Cnmim]+ leads to higher viscosity (η) of RTILs (η[C2mim][NTf2] < η[C4mim][NTf2] < η[C8mim][NTf2]), which is impeditive to both of the cation exchange and the neutral complex extraction.31 Therefore, in the same condition, the DEu decreases with the length of alkyl chain increasing and the BTPhen/[C2mim][NTf2] system shows the best extraction performance of Eu3+. Thus, shorter alkyl chain of [Cnmim][NTf2] or lower [HNO3] are favourable for the effective extraction of Eu3+ in BTPhen/[Cnmim][NTf2] systems. Combined with [C2mim][NTf2], BTPhen could provide satisfactory extraction of Eu3+ at low [HNO3] (0.01 M to 0.1 M HNO3). Additionally, the DEu of BTPhen/1-octanol system was measured under the same conditions for comparison (data refer to Table S2†). BTPhen/1-octanol system achieves the highest DEu (2.56) at 3 M HNO3. BTPhen/[Cnmim][NTf2] system achieved the highest DEu (52) at 0.01 M HNO3 when [C2mim][NTf2] is used. Thus the diluent has obvious different influence on Eu3+ extraction in the presence of nitric acid.
DEu − log[BTPhen] curves in three kinds of [Cnmim][NTf2]. The slope value gradually decreases with the length of alkyl chain increasing, indicating the restraining of the cation exchange mechanism. Thus, longer alkyl chain is less favourable to the cation exchange, due to higher hydrophobicity of the corresponding [Cnmim]+. Furthermore, longer alkyl chain of [Cnmim]+ leads to higher viscosity (η) of RTILs (η[C2mim][NTf2] < η[C4mim][NTf2] < η[C8mim][NTf2]), which is impeditive to both of the cation exchange and the neutral complex extraction.31 Therefore, in the same condition, the DEu decreases with the length of alkyl chain increasing and the BTPhen/[C2mim][NTf2] system shows the best extraction performance of Eu3+. Thus, shorter alkyl chain of [Cnmim][NTf2] or lower [HNO3] are favourable for the effective extraction of Eu3+ in BTPhen/[Cnmim][NTf2] systems. Combined with [C2mim][NTf2], BTPhen could provide satisfactory extraction of Eu3+ at low [HNO3] (0.01 M to 0.1 M HNO3). Additionally, the DEu of BTPhen/1-octanol system was measured under the same conditions for comparison (data refer to Table S2†). BTPhen/1-octanol system achieves the highest DEu (2.56) at 3 M HNO3. BTPhen/[Cnmim][NTf2] system achieved the highest DEu (52) at 0.01 M HNO3 when [C2mim][NTf2] is used. Thus the diluent has obvious different influence on Eu3+ extraction in the presence of nitric acid.
 | ||
Fig. 5 Variations in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEu as a function of log[BTPhen] in [C2mim][NTf2] (a), [C4mim][NTf2] (b), [C8mim][NTf2] (c). Initial nitric acid concentration: 0.1 M. DEu as a function of log[BTPhen] in [C2mim][NTf2] (a), [C4mim][NTf2] (b), [C8mim][NTf2] (c). Initial nitric acid concentration: 0.1 M. | ||
3.2. Radiation effect on BTPhen/[C2mim][NTf2] system
The influence of γ-radiation on the extraction of Eu3+ by BTPhen/[C2mim][NTf2] system at 0.1 M HNO3 is shown in Fig. 6(a). The DEu of the irradiated BTPhen/[C2mim][NTf2] system evidently rises with dose increasing in the presence of 0.1 M HNO3. Such tendency is neither reported nor explicable. The irradiated samples were washed with water before extraction. The consequent DEu is shown in Fig. 6(b) and almost be constant to that in the unirradiated BTPhen/[C2mim][NTf2]. Therefore, the abnormal increase of Eu3+ partitioning of irradiated BTPhen/[C2mim][NTf2] was attributed to some water-soluble radiolytic product. Moreover, it was observed that the influence of γ-radiation on the extraction of Eu3+ in BTPhen/[C2mim][NTf2] system could be eliminated by enhancing the concentration of HNO3 during the extraction. At 1 M HNO3, the DEu of irradiated BTPhen/[C2mim][NTf2] system changes slightly with the increase of dose (Fig. 6(c)). The above results indicate that BTPhen/[C2mim][NTf2] system has high radiation stability.In order to further evaluate the radiation stability of BTPhen/[C2mim][NTf2], the irradiated BTPhen in solid state was also studied by 1H NMR (Fig. S2†) and Micro-FTIR (Fig. S3†). Both 1H NMR and Micro-FTIR spectra of the irradiated samples display slightly change compared with those of the unirradiated samples. Thus BTPhen has satisfactory radiation stability in the solid state due to its relatively stable alkyl side chains and aromatic structure which can dissipate the energy during the irradiation. Furthermore, when the irradiated BTPhen was dissolved in [C2mim][NTf2], the irradiation of BTPhen has no obvious effect on the DEu (the results are omitted since they are similar with Fig. 6(b) and (c) at 0.1 M and 1 M HNO3, respectively). However, when BTPhen was dissolved in 1-octanol, the radiation effect on the extractability of BTPhen/1-octanol system was significant. The DEu of BTPhen/1-octanol system decreases rapidly with the dose increasing (Fig. S4†). According to previous research,11 traditional solvents are easy to decompose and produce active radicals during irradiation. Those radicals can induce serious radiolysis of extractants, which lead to the decrease of extractability. Although BTPhen has great radiation stability in solid state, its extractability is reduced significantly by radiation when it is dissolved in 1-octanol. Apparently, the traditional solvent that easily produces active species during irradiation (e.g. 1-octanol) is very unfavourable for the radiation stability of BTPhen. On the contrary, RTILs are more stable than traditional solvents during irradiation.18 Due to the high viscosity of RTILs, the diffusion rate and activity of radicals produced from solvent are reduced, and the possibility of radiolysis of extractant decreased further.32,33 Thus the radiolysis of BTPhen in [Cnmim][NTf2] is much lower than that in traditional solvents.
Based on the washing-off effect, the increase of DEu is attributed to some water-soluble radiolytic products of the extraction system. In order to elucidate the composition of water-soluble radiolytic products, the removal of Eu3+ by irradiated [C2mim][NTf2] alone was measured at 0.1 M HNO3. The removal of Eu3+ increases with the increase of dose for the irradiated [C2mim][NTf2], which has similar relationship with that for the irradiated BTPhen/[C2mim][NTf2] system. Since the unirradiated [C2mim][NTf2] cannot extract Eu3+ and the irradiated BTPhen slightly affects the extraction of Eu3+, these results indicate that the water-soluble radiolytic products are derived from irradiated [C2mim][NTf2]. We find that when the concentration of Eu3+ is enhanced to 50 mM, visible white sediment is observed at the interface between irradiated [C2mim][NTf2] and the 50 mM Eu3+ solution. The solubility of this sediment is gradually enhanced with the acidity increasing, and the sediment is completely dissolved in 1 M HNO3. This phenomenon indicates that the white sediment is responsible for the abnormal increase of Eu3+ partitioning of the irradiated BTPhen/[C2mim][NTf2] system at 0.1 M HNO3.
Based on 19F NMR analysis of irradiated [C2mim][NTf2] (Fig. S5†), several dominant radiolytic products (HF, CF3SOOH, CF3SOONH2, etc.) are identified based on our previous work.25 A third-phase was observed when the concentration of Eu3+ was enhanced to 50 mM. After a separation procedure from the mixture of 50 mM Eu3+ solution and irradiated [C2mim][NTf2], white powder sediment was obtained and analysed by XPS. The composition of the sediment was determined to contain C, F, O, S and Eu elements according to the analysis of XPS pattern (Fig. 7). The C element is assigned to hydrocarbons which are used for calibration. The BE of elements can be used to identify the specific chemical bonding in different compounds,34 so the core level BE and the BE difference for the obtained sediment are compared with that for Eu2(SO3)3 crystal and EuF3 crystal (Table S3†). The sediment shows strong signal of F element, and a chemical formula of EuF3 for the sediment is confirmed by the atom ratio of F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu which equals to 2.98
Eu which equals to 2.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Apparently, EuF3 is the main species in sediment. Additionally, SO32− has been identified as one of the radiolytic products of [C4mim][NTf2] under γ-irradiation,35 so SO32− could be formed during the irradiation of [C2mim][NTf2] and precipitated with Eu3+ as well. It is found that O and S elements have weak signal in XPS spectrum and can be assigned to Eu2(SO3)3, but they are not main components of the sediment (less than 10%). Therefore, the precipitation between Eu3+ and radiolytic products of [Cnmim][NTf2] (F− and SO32−) leads to the decrease of Eu3+ concentration in water phase. Additionally, the amount of radiolytic products in [Cnmim][NTf2] is very low based on our previous analysis,25 so BTPhen/[C2mim][NTf2] system has good radiation stability during the extraction of Eu3+.
1. Apparently, EuF3 is the main species in sediment. Additionally, SO32− has been identified as one of the radiolytic products of [C4mim][NTf2] under γ-irradiation,35 so SO32− could be formed during the irradiation of [C2mim][NTf2] and precipitated with Eu3+ as well. It is found that O and S elements have weak signal in XPS spectrum and can be assigned to Eu2(SO3)3, but they are not main components of the sediment (less than 10%). Therefore, the precipitation between Eu3+ and radiolytic products of [Cnmim][NTf2] (F− and SO32−) leads to the decrease of Eu3+ concentration in water phase. Additionally, the amount of radiolytic products in [Cnmim][NTf2] is very low based on our previous analysis,25 so BTPhen/[C2mim][NTf2] system has good radiation stability during the extraction of Eu3+.
4. Conclusions
The extraction performance and radiation stability of BTPhen/[Cnmim][NTf2] system during the removal of Eu3+ from HNO3 solution were reported for the first time. The BTPhen/[C2mim][NTf2] system shows the excellent extraction ability of Eu3+ at [HNO3] ≤ 0.1 M. The molar ratio of complexes between BTPhen and Eu3+ changes from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gradually, indicating that the dominant extraction mechanism varies from cation exchange mechanism to neutral species mechanism with the [HNO3] or the length of alkyl chain in the [Cnmim]+ increasing. Although the precipitation between Eu3+ and radiolytic products of [C2mim][NTf2] (F− and SO32−) leads to the increment in removal of Eu3+ for the irradiated BTPhen/[C2mim][NTf2] system at [HNO3] ≤ 0.1 M, the influence of radiolytic products of [C2mim][NTf2] can be easily avoided by enhancing the acidity during extraction or washing the irradiated samples with water before extraction. γ-radiation has no obvious effect on the extraction performance of BTPhen/[C2mim][NTf2] system in the presence of 1 M nitric acid. Therefore, the BTPhen/[C2mim][NTf2] system shows excellent extraction capacity and high radiation stability.
1 gradually, indicating that the dominant extraction mechanism varies from cation exchange mechanism to neutral species mechanism with the [HNO3] or the length of alkyl chain in the [Cnmim]+ increasing. Although the precipitation between Eu3+ and radiolytic products of [C2mim][NTf2] (F− and SO32−) leads to the increment in removal of Eu3+ for the irradiated BTPhen/[C2mim][NTf2] system at [HNO3] ≤ 0.1 M, the influence of radiolytic products of [C2mim][NTf2] can be easily avoided by enhancing the acidity during extraction or washing the irradiated samples with water before extraction. γ-radiation has no obvious effect on the extraction performance of BTPhen/[C2mim][NTf2] system in the presence of 1 M nitric acid. Therefore, the BTPhen/[C2mim][NTf2] system shows excellent extraction capacity and high radiation stability.
Acknowledgements
The National Natural Science Foundation of China (NNSFC, Project no. 21073008, 91126014 and 11079007) is acknowledged for supporting this research.Notes and references
- A. Rout, E. R. Souza and K. Binnemans, RSC Adv., 2014, 4, 11899–11906 RSC.
- A. Sengupta, P. K. Mohapatra, M. Iqbal, W. Verboom, J. Huskens and S. V. Godbole, RSC Adv., 2012, 2, 7492–7500 RSC.
- X. Q. Sun, H. M. Luo and S. Dai, Chem. Rev., 2012, 112, 2100–2128 CrossRef CAS PubMed.
- C. Ekberg, A. Fermvik, T. Retegan, G. Skarnemark, M. R. S. Foreman, M. J. Hudson, S. Englund and M. Nilsson, Radiochim. Acta, 2008, 96, 225–233 CrossRef CAS.
- A. Geist, C. Hill, G. Modolo, M. R. S. J. Foreman, M. Weigl, K. Gompper and M. J. Hudson, Solvent Extr. Ion Exch., 2006, 24, 463–483 CrossRef CAS.
- M. G. B. Drew, M. R. S. J. Foreman, C. Hill, M. J. Hudson and C. Madic, Inorg. Chem. Commun., 2005, 8, 239–241 CrossRef CAS PubMed.
- M. J. Hudson, C. E. Boucher, D. Braekers, J. F. Desreux, M. G. B. Drew, M. R. S. Foreman, L. M. Harwood, C. Hill, C. Madic, F. Marken and T. G. A. Youngs, New J. Chem., 2006, 30, 1171–1183 RSC.
- B. B. Beele, E. Rudiger, F. Schworer, U. Mullich, A. Geist and P. J. Panak, Dalton Trans., 2013, 12139–12147 RSC.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, J. F. Desreux, G. Vidick, N. Bouslimani, G. Modolo, A. Wilden, M. Sypula, T.-H. Vu and J.-P. Simonin, J. Am. Chem. Soc., 2011, 133, 13093–13102 CrossRef CAS PubMed.
- E. Aneheim, C. Ekberg, A. Fermvik, M. R. S. J. Foreman, B. Grűner, Z. Hájková and M. Kvičalová, Solvent Extr. Ion Exch., 2011, 29, 157–175 CrossRef CAS.
- A. Fermvik, L. Berthon, C. Ekberg, S. Englund, T. Retegan and N. Zorz, Dalton Trans., 2009, 6421–6430 RSC.
- M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391–1398 CrossRef CAS.
- V. A. Cocalia, K. E. Gutowski and R. D. Rogers, Coord. Chem. Rev., 2006, 250, 755–764 CrossRef CAS PubMed.
- F. Endres and S. Z. El Abedin, Phys. Chem. Chem. Phys., 2006, 8, 2101–2116 RSC.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- D. Allen, G. Baston, A. E. Bradley, T. Gorman, A. Haile, I. Hamblett, J. E. Hatter, M. J. F. Healey, B. Hodgson, R. Lewin, K. V. Lovell, B. Newton, W. R. Pitner, D. W. Rooney, D. Sanders, K. R. Seddon, H. E. Sims and R. C. Thied, Green Chem., 2002, 4, 152–158 RSC.
- L. Berthon, S. I. Nikitenko, I. Bisel, C. Berthon, M. Faucon, B. Saucerotte, N. Zorz and P. Moisy, Dalton Trans., 2006, 2526–2534 RSC.
- I. A. Shkrob, S. D. Chemerisov and J. F. Wishart, J. Phys. Chem. B, 2007, 111, 11786–11793 CrossRef CAS PubMed.
- S. Dai, Y. H. Ju and C. E. Barnes, J. Chem. Soc., Dalton Trans., 1999, 1201–1202 RSC.
- A. Sengupta, P. K. Mohapatra, M. Iqbal, J. Huskens and W. Verboom, Dalton Trans., 2012, 6970–6979 RSC.
- A. B. Patil, P. Pathak, V. S. Shinde, S. V. Godbole and P. K. Mohapatra, Dalton Trans., 2013, 1519–1529 RSC.
- M. Steppert, I. Cisarova, T. Fanghanel, A. Geist, P. Lindqvist-Reis, P. Panak, P. Stepnicka, S. Trumm and C. Walther, Inorg. Chem., 2012, 51, 591–600 CrossRef CAS PubMed.
- G. Benay, R. Schurhammer and G. Wipff, Phys. Chem. Chem. Phys., 2011, 13, 2922–2934 RSC.
- G. Benay and G. Wipff, J. Phys. Chem. B, 2013, 117, 1110–1122 CrossRef CAS PubMed.
- Y. Y. Ao, J. Peng, L. Y. Yuan, Z. P. Cui, C. Li, J. Q. Li and M. L. Zhai, Dalton Trans., 2013, 4299–4305 RSC.
- I. Billard, A. Ouadi and C. Gaillard, Anal. Bioanal. Chem., 2011, 400, 1555–1566 CrossRef CAS PubMed.
- A. Rout, K. A. Venkatesan, T. G. Srinivasan and P. R. V. Rao, J. Hazard. Mater., 2012, 221, 62–67 CrossRef PubMed.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, P. Distler, J. John, K. Stamberg, A. Núñez, H. Galán and A. G. Espartero, Eur. J. Org. Chem., 2012, 1509–1519 CrossRef CAS.
- I. Billard, A. Ouadi and C. Gaillard, Dalton Trans., 2013, 6203–6212 RSC.
- A. Rout, S. Karmakar, K. A. Venkatesan, T. G. Srinivasan and P. R. V. Rao, Sep. Purif. Technol., 2011, 81, 109–115 CrossRef CAS PubMed.
- H. Tokuda, S. Tsuzuki, M. A. B. H. Susan, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2006, 110, 19593–19600 CrossRef CAS PubMed.
- D. Behar, C. Gonzalez and P. Neta, J. Phys. Chem. A, 2001, 105, 7607–7614 CrossRef CAS.
- S. Sarkar, S. Mandal, C. Ghatak, V. G. Rao, S. Ghosh and N. Sarkar, J. Phys. Chem. B, 2012, 116, 1335–1344 CrossRef CAS PubMed.
- P. van der Heide, X-ray Photoelectron Spectroscopy: An introduction to Principles and Practices, Wiley, 2011 Search PubMed.
- L. Y. Yuan, C. Xu, J. Peng, L. Xu, M. L. Zhai, J. Q. Li, G. S. Wei and X. H. Shen, Dalton Trans., 2009, 7873–7875 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Influence of the oscillation time on EEu in BTPhen/[C2mim][NTf2] system, the data of DEu in BTPhen/[Cnmim][NTf2] systems depending on the initial nitric acid concentration, the data of DEu in BTPhen/1-octanol system depending on the initial nitric acid concentration, 1H NMR spectra of BTPhen before and after irradiation, Micro-FTIR spectra of BTPhen before and after irradiation, 19F NMR spectra of [C2mim][NTf2] before and after irradiation, core level binding energy (sediment, Eu2(SO3)3 and EuF3). See DOI: 10.1039/c4ra07662a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |




