Red Mud waste from the Bayer process as a catalyst for the desulfurization of hydrocarbon fuels†
Abstract
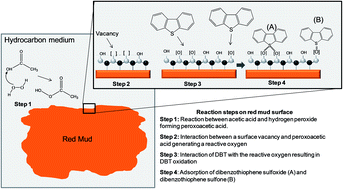
The management of Red Mud generated as a waste by-product of bauxite processing in the aluminum industry is key to the long-term sustainability of alumina production. At the same time, the desulfurization of fuel oil to low-level sulfur is an ongoing challenge to the petroleum industry. In an attempt to address both these issue in an integrated fashion, Red Mud waste was studied as a catalyst in a desulfurization process using a simulated Diesel containing dibenzothiophene (DBT) as a model heterocyclic organic sulfur compound. The new process combines an oxidative-adsorptive desulfurization in which a combination of H2O2//H3CCOOH and Red Mud was able to catalytically oxidize the sulfur compound to the corresponding sulfoxide (DBTO) and sulfone (DBTO2) which are then reversibly adsorbed onto the Red Mud. Regenerative tests of Red Mud were performed, maintaining a high activity in recycles suggesting that the Red Mud could be reused after a simple thermal treatment releasing DBTO and DBTO2. The approach synergistically addresses both problems: removal of sulfur compounds while at the same time offering a potential useful application of Red Mud. Red Mud was characterized by Mössbauer Spectroscopy, Infrared Spectroscopy, X-ray Diffraction (XRD), Scanning Electron Microscopy with dispersive energy (SEM-EDS), BET Surface area (BET), and release of volatile compounds from Red Mud by Gas chromatography-Mass Spectrometry (GC-MS) using a solvent-free solid injector (SFSI). The kinetics of the desulfurization reactions were monitored by GC-MS.

 Please wait while we load your content...
Please wait while we load your content...