3-Aminopropyltrimethoxysilane and organic electron donors mediated synthesis of functional amphiphilic gold nanoparticles and their bioanalytical applications†
Abstract
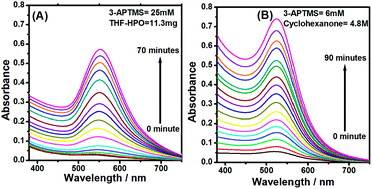
Precise control over functionality and nanogeometry during synthesis of gold nanoparticles (AuNPs) and dispersibility of the same in a variety of solvents has been a challenging requirement for practical applications. Organic reducing agents i.e. 3-glycidoxypropyltrimethoxysilane (3-GPTMS), tetrahydrofuran hydroperoxide (THF–HPO) and cyclohexanone have shown potential for meeting these requirements during the conversion of 3-aminopropyltrimethoxysilane (3-APTMS)-capped gold ions into AuNPs. The reaction products of these reducing agents with 3-APTMS during AuNPs synthesis are catalytic in nature for THF–HPO and cyclohexanone due to the formation of inorganic–organic hybrids and non-catalytic for 3-GPTMS. The presence and absence of such reaction products justify the difference in catalytic ability of the AuNPs as a function of organic reducing agents, which profusely affects the inherent properties of AuNPs for specific applications. The use of cyclohexanone in place of 3-GPTMS or THF–HPO together with 3-APTMS outclasses the other two in imparting better stability to amphiphilic AuNPs with reduced silanol content. The as-synthesized AuNPs enable the formation of nanocomposite (PBNPs–AuNPs) dispersion with Prussian blue nanoparticles (PBNPs). Further, PBNPs–AuNPs may also be converted to homogeneous nanocomposite suspensions with ruthenium bipyridyl solution, justifying even better catalytic ability than that of HRP. The resulting nanomaterial suspensions in one way, efficiently probe the glucose oxidase catalyzed reactions based on peroxidase mimetic ability and, in another way, display excellent electrocatalytic activity during the electrochemical sensing of H2O2. The peroxidase mimetic ability of the nanomaterials has been found to vary as a function of 3-APTMS concentration, which confirms the potential role of functional gold nanoparticles in bioanalytical applications.

 Please wait while we load your content...
Please wait while we load your content...