One-pot solvent-free reductive amination with a solid ammonium carbamate salt from CO2 and amine†
Abstract
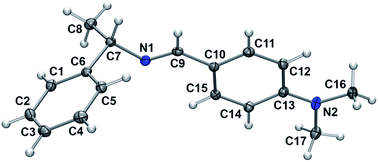
Many amines are liquid and their handling is inconvenient compared with the corresponding solids. We transformed a liquid (S)-(−)-1-phenylethylamine 1 to the corresponding neutral solid form 2 by reacting with carbon dioxide. We performed reductive amination of 2 with various aldehydes 3 under solvent-free conditions to provide secondary amines 5 in high yields.

 Please wait while we load your content...
Please wait while we load your content...