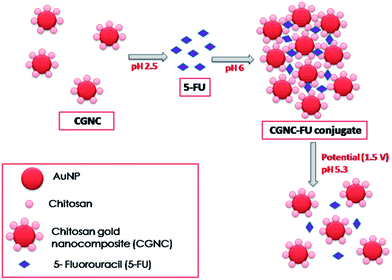
An electric field responsive drug delivery system based on chitosan–gold nanocomposites for site specific and controlled delivery of 5-fluorouracil†
Parvathy R. Chandran and
N. Sandhyarani*
Nanoscience Research Laboratory, School of Nano Science and Technology, National Institute of Technology, Calicut, Kerala, India. E-mail: sandhya@nitc.ac.in; Fax: +91 495 2287250; Tel: +91 495 2286537
First published on 9th September 2014
Abstract
A novel drug delivery system based on an electric field and pH dual-stimuli responsive chitosan–gold nanocomposite (CGNC) is reported in the present study for site specific controlled delivery of the anticancer drug 5-fluorouracil (5-FU). At a higher pH of the solution CGNC encapsulates the drug molecules in its 3D-network and releases the drug at a relatively lower pH due to its reversible gel–sol transition properties. A sulphorhodamine B cytotoxicity assay showed that the chitosan–gold nanocomposite–fluorouracil conjugate (CGNC–FU) has higher cytotoxicity than 5-FU to the cervical cancer cells, SiHa. Drug release from CGNC has been controlled by applying a weak, external DC electric field. The drug delivery system is illustrated to be effective for the loading and delivery of 5-FU. The encapsulation efficiency of CGNC was found to be 36% and about 63% of the drug was released by applying the electric field. The biocompatibility of CGNC was demonstrated by growing SiHa cells successfully on a CGNC film electrodeposited on an indium tin oxide (ITO) coated glass plate. SiHa cells grown on a CGNC–FU conjugate modified ITO plate showed less viability and further application of an electric field of 1.5 V for the release of 5-FU led to the complete death of the cells. A highlight of the present drug delivery system is that the drug release can be controlled externally.
Introduction
Nanobiotechnology offers new strategies for solving the problems encountered with cancer drug delivery. Researchers focus on developing stimuli-responsive smart materials for delivering drugs to the target site in order to prevent the shortcomings in conventional cancer therapy such as poor bioavailability of drug, side effects of drug, etc. Recently various polymeric nanoparticles and metallic nanoparticles have gained attention as stimuli responsive materials. Heat,1 light,2 pH,3 enzymes,4 ultrasonic waves5 and magnetic fields6 are reported as stimuli to trigger the release of drug from the carrier molecule in a controlled way. By tailoring drug release kinetics, the side effects of the anticancer drugs can be reduced. Since cancer cells show a lower pH than the normal cells,7 pH responsive drug release is a far and wide approach used in cancer drug delivery.8–10Due to its characteristic optical and chemical properties, gold nanoparticles have been widely explored in different biomedical applications such as bioimaging and sensing,11,12 diagnosis,13 targeting and drug delivery.14 Recently gold nanoparticles were designed for dynamic X-ray imaging of blood flows by taking advantage of their X-ray absorption properties.15 They have used red blood cells as carrier of the contrast agents for the blood flow studies. Gold nanorods, nanocages and nanoshells have been used for photothermal therapy.16–19 Gold nanoparticles are also been used in drug delivery applications by conjugating them to drug molecules through pH labile20,21 and photo labile linkers.22
Intrinsically conducting polymers are reported to be smart materials for electric field triggered release of drug molecules.23,24 Ge et al., in 2012 developed a new temperature and electric field dual-stimuli responsive nanoparticle system for programmed drug delivery.25 They have encapsulated fluorescein molecule in conductive polymer polypyrrole nanoparticles utilizing emulsion polymerization technique and illustrated the electric field responsive release with time.
Chitosan, a common biopolymer prepared by the deacetylation of chitin, has been explored extensively for biomedical applications. Biodegradability, non toxicity and biocompatibility render chitosan a common carrier for drug,26 gene27,28 and vaccine.29 Different models of chitosan based drug delivery systems are explored using different anticancer drugs.30,31 Like many other biopolymers, chitosan is also used as a stabilizing agent for the synthesis of gold nanoparticles32,33 and chitosan protected gold nanoparticles are proved as one of the best candidates for biological applications.34,35 Bhumkar et al., used chitosan reduced gold nanoparticles as carrier systems for insulin delivery.36 Chitosan gold nanoparticles attached with small hairpin RNA have shown to be well internalized in the cell and exhibited good gene silencing effects.37 Guo et al., have studied the multifunctional potentiality of chitosan–gold nanorod hybrid structures for cell imaging, drug delivery and photothermal therapy.38 In all these studies they have used the electrostatic interaction between the chitosan matrix and drug molecule for the drug loading. Interestingly, we observed that chitosan gold nanocomposites (CGNC) exhibit significant pH sensitivity.39 Amino group in chitosan, with a pKa value of ∼6.5, is positively charged and soluble in acidic to neutral solution, converts to a less stable amorphous form at higher pH and these changes were found to be reversible. Thus at higher pH they will form a three dimensional network enabling the encapsulation of small biomolecules. This property of CGNC has been exploited for drug delivery application, particularly for cancer treatment.
In the present drug delivery system, the anticancer drug 5-fluorouracil (5-FU) was treated with chitosan–gold nanocomposite (CGNC) at pH 2.5. Then pH of the solution was adjusted to 6–6.5 to achieve the encapsulation of drug molecules into the gel structures as illustrated in Scheme 1. Drug release in solution was mediated through the change in pH (pH was lowered down to 5.3 as there is a change in pH in the cancer cell microenvironment). Under the low pH, gel–sol transition takes place releasing the drug. When electric field was applied, this lowers the pH around the electrode to about 4.7 to 5 and released the drug more effectively with external control. The system is demonstrated to be as an excellent carrier for externally controlled site specific drug delivery. Though chitosan coated gold nanoparticle mediated drug delivery is widely explored, the controlled delivery of drugs with the application of electric field is reporting for the first time. Gold nanoparticles are incorporated into chitosan owing to their high electrical conducting property. This approach proposes that by implanting an electrode deposited with CGNC–FU near to the cancer cells and by applying weak electric field, the drug release can be controlled externally.
Experimental section
1.1 Materials
Chloroauric acid and sodium borohydride were purchased from Sisco Research Laboratories, India and were used as received. Acetic acid and sodium hydroxide were purchased from Merck International. Biomedical grade chitosan was obtained from the Central Institute of Fisheries Technology, Cochin, India. Chitosan had a molecular weight of 270 kDa, and degree of deacetylation was 85%. This was used as received. 5-Fluorouracil and sulphorhodamine B were purchased from Sigma-Aldrich. Minimal Essential Media (MEM) was purchased from Himedia, Mumbai, India. Indium Tin Oxide (ITO) coated glass plate with a surface resistivity of 70–100 Ω sq−1 was purchased from Sigma-Aldrich. Ultrapure water (DI water, 18 MΩ cm) was used for all experiments.1.2 Synthesis of chitosan–gold nanocomposite (CGNC)
CGNC was synthesized following a modified procedure.39 In a typical experiment, 10 ml of 1% (w/v) chitosan solution was prepared in 20% (v/v) acetic acid. The pH of the solution was maintained at 2.5. About 25 ml of 5 mM chloroauric acid solution was added to the vigorously stirred solution of chitosan. The resulting mixture was stirred for 1 hour, and then 2 ml of freshly prepared 10 mM sodium borohydride was added dropwise. The formation of gold nanoparticles was manifested by the development of a ruby red color in the solution. Stirring was continued for another 2 hours. After synthesis CGNC was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 minutes to remove unreacted chitosan.
000 rpm for 30 minutes to remove unreacted chitosan.
1.3 Characterization of chitosan–gold nanocomposite (CGNC)
All measurements were performed at room temperature. Absorption spectra were recorded with a UV-1800 Shimadzu double beam spectrophotometer. IR spectroscopic measurements were performed with a Perkin Elmer Spectrum 400 FT-IR spectrometer. Transmission Electron Microscope (TEM) images were taken using Philips CM200 Transmission Electron Microscope working at an acceleration voltage of 80 keV. Samples were prepared by drop-casting and drying 10 μl of sample solution on carbon-coated copper grids. Atomic Force Microscopy (AFM) was performed using Park Systems XE-100 AFM. The size distribution and zeta potential of CGNC were evaluated by dynamic light scattering (DLS) measurement at 25 °C using a Dynamic laser scattering system, Zetasizer Nano ZS (Malvern, UK). Bright field optical images were taken using Olympus BX-51 optical microscope equipped with Olympus DP 72 digital camera and Scanning Electron Microscope (SEM) images were taken using Hitachi SU6600 Scanning Electron Microscope.1.4 Drug encapsulation and determination of encapsulation efficiency
To 3 ml of the CGNC solution at pH 2.5, 1 ml of 10 mM 5-FU was added. The pH of the solution was then changed to 6 by adding 3 M NaOH. The CGNC–FU conjugate was purified by centrifugation at 5000 rpm for 15 minutes to remove the free drug. The pellet obtained was washed several times with DI water. The purified drug conjugate was dispersed in 1 ml acetic acid (pH 2) to release the entire encapsulated drug and centrifuged at 12000 rpm for 20 minutes. The supernatant was then analyzed by UV-visible absorption spectrum to determine the encapsulation efficiency of CGNC. Absorbance at 266 nm was recorded and calculated the encapsulation efficiency using the formula.1.5 Modification of ITO/gold electrode with CGNC and CGNC–FU
ITO plates or gold plates were used as electrodes for the deposition of CGNC–FU and for the application of electric field to release the drug 5-FU. In this report we describe the experiments with ITO electrodes only as ITO electrodes were used for optical microscope imaging utilizing its transparency to light. For the electric field assisted drug release studies, the ITO plate was modified by drop casting CGNC or CGNC–FU and applied electric field as discussed in the text. For the optical imaging studies, to avoid background interference, a thin layer of CGNC or CGNC–FU was electrodeposited instead of dropcasting. For electrodeposition, CGNC/CGNC–FU conjugate at a pH 2.5 was used as the electrolyte. A potential of 1.5 V was applied for 2 minutes by a DC power supply using cleaned ITO plates as anode and cathode. A thin film of CGNC/CGNC–FU gets electrodeposited at the cathode. The potential and time for electrodeposition was optimized by doing electrodeposition at various potentials viz., 0.5 V, 1 V, 1.5 V and 3 V at various time durations 1 minute, 2 minutes, 3 minutes or 5 minutes.391.6 Drug release study
Drug release from CGNC–FU conjugate is due to the decrease in pH and further dissolution of CGNC. To tailor the drug release in a controlled manner and to increase the drug release percentage an electric field has been applied. Electric field assisted drug release was monitored by applying an external electric field as triggering factor. The CGNC–FU conjugate modified ITO electrode was used as the anode. A clean ITO plate was used as the cathode. The two electrodes were dipped in 15 ml distilled water at 37 °C. The pH was adjusted to 5.3 using 0.1 N HCl, as the use of buffers interfered with the absorbance peak of 5-FU. The electrolyte was stirred continuously and applied a potential of 1.5 V using a DC power supply for 15 minutes with 1 h interval upto 5 hours. On applying potential, pH around the anode decreases to 4.7 to 5 resulting in the conversion of gel to sol form of chitosan which leads to the release of the encapsulated drug. The drug release was confirmed by UV-Vis absorption peak of the electrolyte at 266 nm and the spectrum was analyzed for quantitative estimation of 5-FU at selected time intervals. Absorbance at 266 nm was noted to calculate the amount of drug released. Control experiments were carried out without applying electric field. The experiments were carried out at two different pH conditions at pH 5.3 and 7.4 (pH adjusted using 0.1 N HCl) with and without the stimuli. In all the experiments the drug release was monitored for about 5 hours as there was no considerable increase in drug release percentage after 5 hours. For baseline correction CGNC without drug was also set aside at the same conditions and the absorbance at 266 nm was subtracted to avoid the interference with the absorbance of released drug.1.7 Cytotoxicity assay (sulphorhodamine B assay)
Human cervical cancer cell lines SiHa were grown in MEM supplemented with 10% (v/v) fetal bovine serum and sodium bicarbonate (1.5 mg ml−1) in the presence of antibiotics (100 units of penicillin, 0.1 mg of streptomycin, 0.25 μg of amphotericin B per ml) at 37 °C in a humidified atmosphere of 5% CO2. Cells were seeded at a density of 5 × 103 cells per well in 96 well tissue culture plate and incubated for 24 hours. After 24 hours incubation, the media was replaced with fresh media and added 100 μl media mixed with the samples-CGNC–FU, CGNC and 5-FU at various concentrations. After 48 hours, cells were fixed with ice cold trichloroacetic acid (25 μl of 50% v/v) at 4 °C for 1 hour, washed four times with tap water, air dried and stained with 100 μl sulphorhodamine B (SRB) reagent (0.4% in 1% acetic acid) for 30 minutes. Unbound SRB was washed out with 1% acetic acid four times, dried and added 150 μl 10 mM tris base (pH 10) to dissolve bound SRB. Absorbance at 550 nm was measured using a microplate reader.40 At least eight repeats were done in each plate for each concentration and the independent assays were repeated for at least three times.1.8 Cell imaging
To study the effect of CGNC and CGNC–FU on growth of cells and to study the effect of electric field on drug release and its toxicity to cells, SiHa cells were grown on Indium Tin Oxide (ITO) coated glass plate modified with CGNC or CGNC–FU, and imaged using bright field optical microscopy and Scanning Electron Microscopy. A control was also made by growing SiHa cells on bare ITO plate. SiHa cells were seeded on the modified and control ITO plates at a density of 5000 cells. A uniform distribution of cells on the surface of the plates was accomplished by placing a circular mould (diameter 2 cm and height 0.5 cm) made of poly dimethyl sulphoxide (PDMS) on the ITO plate and the ITO plate was kept inside a 60 mm sterile petri dish. A schematic representation of the preparation of modified electrode is shown in ESI (Fig. S1†). The cells were grown with 500 μl Minimum Essential Media (MEM) and incubated for 24 h in CO2 incubator. After 12 hours incubation they were washed with PBS and imaged using bright field optical microscopy and SEM. To investigate the effect of electric field assisted drug release on the cells, a potential of 1.5 V was applied for drug release at 37 °C and the morphology of cells were imaged by bright field optical microscopy and SEM.Results and discussion
Earlier we reported that at pH 5–6, CGNC undergoes a sol to gel transition.39 This property has been utilized for encapsulation of the drug 5-FU in the gel matrix and its release in the sol form. To the purified CGNC (which was at a pH of 2–3) drug was added and increased the pH to 6 by adding 3 M NaOH. At this pH, the nanocomposite undergoes a sol to gel like transition encapsulating the drug in the three dimensional network of the composite. The nanocomposite was purified by centrifugation and repeated washing to remove the free drug. The gel like transition and encapsulation of drug was monitored by UV-Vis absorption spectra. Fig. 1 shows the UV-Vis spectra of CGNC before and after encapsulation of drug 5-FU. Before gelation CGNC at pH 2.5 exhibits a plasmon band at 544 nm. For purified CGNC–FU conjugate due to the sol–gel transition of chitosan the plasmon band shifted to 530 nm as reported earlier.39 The observed peak at 248 nm in the spectrum after gelation and purification corresponds to the drug 5-FU which confirms the encapsulation of drug in CGNC. The blue shift in the absorption peak of drug from that of free drug confirms the encapsulation of drug. After the drug release, the absorption peak shifted back to 266 nm, corresponds to the absorption peak of free drug. This shift in absorption maxima of drug confirms the encapsulation and release of 5-FU. Drug encapsulation efficiency was calculated to find out the efficacy of the drug delivery system. The drug encapsulation efficiency can be increased by increasing the amount of drug added. The amount of drug required to attain maximum drug encapsulation was optimized and found that maximum encapsulation efficiency attainable is 36%.Transmission electron micrographs of CGNC at pH 2.7 and CGNC–FU at pH 6 are shown in Fig. 2(a and b). The gold nanoparticles are polydispersed with an average diameter of 20 nm. The particles are almost uniformly dispersed at pH 2.7 and the number of particles per unit area is very less. However, at higher pH 6, as a result of gelation, the number of particles per unit area is increased (For detailed information about the gelation of CGNC, please refer ref. 39). Most of the nanoparticles along with the drug molecules got encapsulated (confirmed from absorption spectrum and IR spectrum) in the chitosan gel matrix.
 | ||
| Fig. 2 TEM image of (A) CGNC at pH 2.7 and (B) CGNC–FU at pH 6 (scale bar: 200 nm). Insets show the images at higher magnification (scale bar: 50 nm). | ||
Fig. 3 shows the atomic force microscopy (AFM) images of CGNC at pH 2.7 and at pH 6. As the figure illustrates, at lower pH the nanoparticles are uniformly dispersed over the region. At pH 6, the particles are clustered together due to the gelation of CGNC with increase in pH. Bright field optical microscopy (ESI† S2) images also confirm the changes due to gelation.
TEM images confirmed that the size of the Au nanoparticles did not change due to the gelation of the CGNC. However, the hydrodynamic radius will change due to the gelation which has been studied using DLS. DLS measurements (Table 1) showed that the hydrodynamic diameter of the nanocomposite increases with the increase in pH. Initially, at a lower pH the amino group in the chitosan is in the protonated form and an increase of pH results in neutralization of amine groups and resultant inter-chain entanglements through hydrogen bonding. Due to this formation of network the hydrodynamic diameter increases which is clear from DLS measurements. The size distribution reports of CGNC (purified after synthesis) and CGNC–FU at pH 6 are shown in ESI (Fig. S3 and S4†). The gelation of nanocomposite was further confirmed by zeta potential measurements. The zeta potential value of CGNC was +32.5 mV at pH 2.7, decreased to +6.21 mV for CGNC–FU at pH 6. The positive charge at lower pH is due to the protonated amino groups of CGNC. As pH increases, amino groups are converted to deprotonated form and zeta potential decreases.
| Sample | Size (nm) | Zeta potential (mV) |
|---|---|---|
| CGNC | 48.6 | +32.5 |
| CGNC–FU pH 6 | 89.8 | +6.21 |
FT-IR confirmed the presence of drug in the CGNC composite (Fig. 4). The spectra of 5-FU and CGNC–FU showed characteristic peak of pyrimidine compound at 1348 cm−1 and C–F stretching band in the region 1000–1400 cm−1. The peak at 760 cm−1 is assigned as due to the pyrimidine ring breathing mode of 5-FU. The intensity of characteristic 5-FU peaks got decreased in the composite suggesting the presence of encapsulated drug. No significant shift is observed in the peaks suggesting the absence of chemical interaction between the FU and CGNC matrix. These changes confirm the encapsulation of the drug into the network of the CGNC gel-like structure.
Drug release has been done at two pH conditions namely at pH 5.3 and at pH 7.4. Fig. 5(A) demonstrates the percentage drug release as a function of time for a period of 5 hours. We noted a higher percentage of drug release at pH 5.3. The drug release at this pH was due to the breaking of gel network and the release of encapsulated drug. It may be noted that the percentage of drug release is only 40–45% since the transformation of gel is not complete at this pH. Though the drug release percentage was high in the lower pH we selected pH 5.3 since the pH near cancer cells are around 5.3. To improve the drug release percentage and to control the drug release we applied an external electric field as stimulus. We noted that on applying potential, pH around the anode further decreases to 4.7 to 5 leads to better dissolution of CGNC gel to sol form resulting in the higher percentage release of the encapsulated drug. Drug release using applied electric field was done by drop casting CGNC–FU onto ITO plate as explained in the experimental section. Fig. 5(B) demonstrates the percentage drug release as a function of time for a period of 5 hours in presence of external stimulus. As shown in Fig. 5, a burst release of 5-FU in the range of 10–15% was noted even in the absence of external stimulus. This initial rapid release may be due to the drug adsorbed on the surface of CGNC, which could be easily released. At pH 5.3 in the presence of external stimulus the burst release is almost 30%. It may be noted that the drug conjugate was purified several times to remove the unbound/free drug molecule. Hence these observations suggest that almost 30% of the drug is either surface adsorbed or loosely encapsulated on CGNC. The sustained release of this weekly bound drug could be attained without the stimulus. However, on application of an external electric field the percentage drug release could be increased to ∼63% in 5 hours. This is due to the further decrease in pH around the electrode surface upon application of a potential and CGNC loses its gel structure releasing the drug (see ESI Fig. S2†). After 5 hours of incubation significant release of drug from CGNC was not observed in all the experiments. 100% drug release could not be obtained due to the incomplete dissolution of the CGNC gel into the releasing media (see optical images in the ESI†).
 | ||
| Fig. 5 Percentage release of 5-FU from CGNC–FU conjugate in the presence and absence of external stimulus at (A) pH 7.4 (B) pH 5.3 as a function of time. | ||
The drug release in the absence of electric field stimulus was about 45% and 21% at pH 5.3 and 7.4 respectively. About 63% of drug was released by applying external stimulus at a pH of 5.3. At the normal physiological pH 7.4 the drug release percentage was about 50% in the presence of stimulus. It may be noted that in addition to the low pH of the electrolyte, the pH in the vicinity of anode was lowered further upon the application of electric field due to the increased H+ ion concentration around anode.39 This reduction in pH leads to the dissolution of CGNC and thereby enhance drug release rate at pH 5.3 in the presence of applied electric field. The application of electric field enhances the percentage drug release and it provides a platform for controlled and sustained release of drug.
The percentage of drug encapsulation and drug release were proved to be adequate to induce toxicity on SiHa cells as confirmed by sulphorhodamine B cytotoxicity assay. Sulphorhodamine B assay was performed to evaluate the cytotoxicity of the drug delivery system. Three samples were tested for cytotoxicity viz. CGNC, CGNC–FU and 5-FU. Results are shown in ESI, Fig. S5.† The assay confirmed that CGNC–FU conjugate exhibited higher toxicity over the drug alone. The cytotoxic activity of the drug is enhanced in the conjugate with an IC50 value of 0.2 μM whereas the drug alone had shown an IC50 of 0.68 μM. Here the enhanced toxicity for CGNC–FU compared to 5-FU alone can be explained as due to the enhanced internalization of CGNC–FU conjugate as such which is mediated through chitosan. It is reported that chitosan enhances the internalization of drug.41 The polycationic nature helps chitosan to quickly bind to negatively charged surfaces such as cellular membranes. Besides, the amine groups make chitosan able to bind to serum proteins and to recognize specific receptors that are present on various types of cancer cells. CGNC–FU conjugate, showing higher toxicity in SRB assay was electrodeposited on electrodes and used for cell imaging studies.
The drug release studies proved the efficiency of the system for electric field responsive controlled drug delivery. To prove the non-toxicity of CGNC and to study the effect of electric field and released drug on the cells, we have grown SiHa cells on ITO plate modified with CGNC and CGNC–FU conjugate. A control was also set by growing SiHa cells on bare ITO plate. Fig. 6 shows the bright field optical images of SiHa cells on ITO plate (control), CGNC coated ITO plate and CGNC–FU coated ITO plate before and after applying electric field. CGNC and CGNC–FU were coated on ITO plate by electrodeposition. Corresponding lower magnification images under 10× objectives are shown in ESI (Fig. S6†) to see cells in a larger area. It is seen that SiHa cells could grow well on the ITO plate and they retain their morphology even after applying electric field. This experiment suggests that the applied electric field (1.5 V) alone do not have any significant effect on the viability of cells. Images shown in Fig. 6(C and D) point out that CGNC provides a biocompatible platform for the growth of cells. However, after applying the electric field few cells lost their morphology indicating the death of cells. As explained earlier, on applying a potential the pH decreases in the vicinity of the electrode and transformation of chitosan starts leading to cell death. The cells that were grown on CGNC–FU conjugate coated ITO plate show substantial death due to the cytotoxic effect of 5-FU. After applying electric field stimulus for drug release, about 95% of the cells were shown to have lost their viability. As per the criteria proposed by nomenclature committee on cell death (NCCD) for the definition of cell death, the cells that has lost the integrity of plasma membrane, nucleus undergoing fragmentation and the fragments being engulfed by adjacent cells are considered as dead cells.42 The cells grown on CGNC–FU are shown to lose their plasma membrane integrity and the nuclei are fragmented after applying electric field. This confirms the efficiency of the drug delivery system. The results are in agreement with SRB assay and drug release studies. These results put forward an idea of implantable drug release system for externally controlled delivery of drug in response to the applied electric field. We are proposing the system for site specific drug delivery specifically on easily accessible surface tumors so that non-tumor cells will not be affected.
 | ||
| Fig. 6 Bright field optical microscope images of SiHa cells grown on modified and control ITO plates before and after applying potential. ‘I’ shows SiHa cells on ITO plate, ‘II’ shows SiHa cells on ITO modified with CGNC and ‘III’ shows SiHa cells on ITO modified with CGNC–FU conjugate (scale bar: 20 μm). SiHa cells grown on CGNC–FU are shown to have lost their viability after applying potential.42 | ||
Change in morphology of SiHa cells was further established by Scanning Electron Microscopy images (Fig. 7). Fig. 7(A) corresponds to SiHa cells grown on ITO plate without CGNC–drug treatment. Fig. 7(B) represents the cells grown on ITO plate electrodeposited with CGNC and 7(C) corresponds to cells grown on ITO plate electrodeposited with CGNC–FU. All the images were taken after applying electric field. In Fig. 7(A) the cells were more confluent when compared to Fig. 7(B) and (C). The cells remain viable in Fig. 7(A) and (B) with definite morphology of SiHa cells. But in Fig. 7(C) the cells are shrunk manifested by volume reduction of nucleus and cytoplasm. The cell membranes have lost their integrity and the nuclei were fragmented confirming cell death. These changes can be attributed to the effect of electric field stimulus to release the drug molecules and thereby causing cell death.
As the pH sensitivity may cause toxicity to the normal tissue, we are suggesting the drug delivery system for site specific drug delivery, especially to the accessible tumors. As CGNC–FU conjugate is more toxic than drug alone, the system can be used for effective cancer therapy. The drug loading into the nanocarrier is by encapsulation without involving complex chemical functionalization. Most of the drug molecule can be encapsulated into the CGNC despite its chemical functionality and can be effectively used for respective therapy which is confirmed by using dacarbazine as the drug. We have seen a comparable performance with dacarbazine too. Here we have selected ITO plate as the electrode for growing cells as it facilitates the optical microscope imaging. As a biocompatible surface we suggest gold electrode for the implantable device. We have carried drug release experiments with gold electrodes as well. The results are similar with that using ITO plate and are shown in ESI (Fig. S7†).
Conclusions
We have developed a novel drug delivery system based on CGNC, which can be used as an implantable device for the externally controlled delivery of drug. In this study 5-FU was used as the model drug. The lower pH in the cancer cell environment acts as the trigger for drug release. Site specific drug delivery can be controlled by lowering the pH in the presence of electric field. Cytotoxicity assay proved that the toxicity to the SiHa cells treated with CGNC–drug conjugate was higher than the cells treated with drug alone. The cells were grown on ITO plate modified with CGNC and CGNC–FU in order to simulate the extracellular condition of cancer cells. The image analysis by bright field optical microscopy and scanning electron microscopy indicated the changes in morphology of the cells confirming cell death after treating with the drug delivery system under applied electric field.Acknowledgements
PRC and NS thank Department of Biotechnology, Government of India for financial assistance through the project BT/PR10820/NNT/28/109/2008. PRC thanks Department of Science and Technology, Government of India for the INSPIRE-JRF fellowship. We thank Dr Rathinasamy K and Miss. Lakshmi Mohan, School of Biotechnology, NIT Calicut for providing SiHa cells and extending cell culture facility. We thank Sophisticated Analytical Instrument Facility (SAIF), Indian Institute of Technology, Mumbai (IITB) for their assistance in TEM measurements.References
- K. S. Soppimath, D. W. Tan and Y. Y. Yang, Adv. Mater., 2005, 17, 318 CrossRef CAS.
- J. You, G. Zhang and C. Li, ACS Nano, 2010, 4, 1033 CrossRef CAS PubMed.
- E. S. Lee, H. Shin, K. Na and Y. H. Bae, J. Controlled Release, 2003, 90, 363 CrossRef CAS.
- K. Patel, S. Angelos, W. R. Dichtel, A. Coskun, Y. W. Yang, J. I. Zink and J. F. Stoddart, J. Am. Chem. Soc., 2008, 130, 2382 CrossRef CAS PubMed.
- G. A. Husseini, K. L. O'Neill and W. G. Pitt, Technol. Cancer Res. Treat., 2005, 4, 707 CAS.
- S. J. Son, J. Reichel, B. He, M. Schuchman and S. B. Lee, J. Am. Chem. Soc., 2005, 127, 7316 CrossRef CAS PubMed.
- P. W. Vaupel, S. Frinak and H. I. Bicher, Cancer Res., 1981, 41, 2008 CAS.
- E. R. Gillies and J. M. Fréchet, Bioconjugate Chem., 2005, 16, 361 CrossRef CAS PubMed.
- R. Liu, Y. Zhang, X. Zhao, A. Agarwal, L. J. Mueller and P. Feng, J. Am. Chem. Soc., 2010, 132, 1500 CrossRef CAS PubMed.
- Q. Yang, S. Wang, P. Fan, L. Wang, Y. Di, K. Lin and X. Feng-Shou, Chem. Mater., 2005, 17, 5999 CrossRef CAS.
- J. Liu and Y. A. Lu, J. Am. Chem. Soc., 2003, 125, 6642 CrossRef CAS PubMed.
- S. O. Obare, R. E. Hollowell and C. J. Murphy, Langmuir, 2002, 18, 10407 CrossRef CAS.
- J. Kneipp, H. Kneipp, M. McLaughlin, D. Brown and K. Kneipp, Nano Lett., 2006, 6, 2225–2231 CrossRef CAS PubMed.
- G. F. Paciotti, L. Myer, D. Weinreich, D. Goia, N. Pavel, R. E. McLaughlin and L. Tamarkin, Drug Delivery, 2004, 11, 169 CrossRef CAS PubMed.
- S. Ahn, S. Y. Jung, E. Seo and S. J. Lee, Biomaterials, 2011, 32, 7191 CrossRef CAS PubMed.
- C. M. Cobley, L. Au, J. Chen and Y. Xia, Expert Opin. Drug Delivery, 2010, 7, 577 CrossRef CAS PubMed.
- L. R. Hirsch, R. Stafford, J. Bankson, S. Sershen, B. Rivera, R. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549 CrossRef CAS PubMed.
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115 CrossRef CAS PubMed.
- T. B. Huff, L. Tong, Y. Zhao, M. N. Hansen, J. X. Cheng and A. Wei, Nanomedicine, 2007, 2, 125 CrossRef CAS PubMed.
- S. Aryal, J. J. Grailer, S. Pilla, D. A. Steeber and S. Gong, J. Mater. Chem., 2009, 19, 7879 RSC.
- P. R. Chandran, P. Appadurai, K. Rathinasamy and N. Sandhyarani, J. Nanopharmaceutics Drug Delivery, 2013, 1, 1 CrossRef PubMed.
- L. Poon, W. Zandberg, D. Hsiao, Z. Erno, D. Sen, B. D. Gates and N. R. Branda, ACS Nano, 2010, 4, 6395 CrossRef CAS PubMed.
- P. M. George, D. A. LaVan, J. A. Burdick, C. Y. Chen, E. Liang and R. Langer, Adv. Mater., 2006, 18, 577 CrossRef CAS.
- D. Svirskis, J. Travas-Sejdic, A. Rodgers and S. Garg, J. Controlled Release, 2010, 146, 6 CrossRef CAS PubMed.
- J. Ge, E. Neofytou, T. J. Cahill III, R. E. Beygui and R. N. Zare, ACS Nano, 2012, 6, 227 CrossRef CAS PubMed.
- Y. Pan, Y.-J. Li, H.-Y. Zhao, J.-M. Zheng, H. Xu and G. Wei, Int. J. Pharm., 2002, 249, 139 CrossRef CAS.
- K. Lee, I. Kwon, Y. H. Kim, W. Jo and S. Jeong, J. Controlled Release, 1998, 51, 213 CrossRef CAS.
- C. Li, T. Guo, D. Zhou, Y. Hu, H. Zhou, S. Wang, J. Chen and Z. Zhang, J. Controlled Release, 2011, 154, 177 CrossRef CAS PubMed.
- L. Illum, I. Jabbal-Gill, M. Hinchcliffe, A. Fisher and S. Davis, Adv. Drug Delivery Rev., 2001, 51, 81 CrossRef CAS.
- M. Razmi, A. Divsalar, A. A. Sboury, Z. Izadi, T. Haertlé and H. Mansuri-Torshizi, Colloids Surf., B, 2013, 112, 362 CrossRef CAS PubMed.
- T. Nam, S. Park, S. Y. Lee, K. Park, K. Choi, I. C. Song, M. H. Han, J. J. Leary, S. A. Yuk, I. C. Kwon, K. Kim and S. Y. Jeong, Bioconjugate Chem., 2010, 21, 578 CrossRef CAS PubMed.
- K. Esumi, N. Takei and T. Yoshimura, Colloids Surf., B, 2003, 32, 117 CrossRef CAS.
- H. Huang and X. Yang, Biomacromolecules, 2004, 5, 2340 CrossRef CAS PubMed.
- M. J. Laudenslager, J. D. Schiffman and C. L. Schauer, Biomacromolecules, 2008, 9, 2682 CrossRef CAS PubMed.
- I. Wedmore, J. G. McManus, A. E. Pusateri and J. B. Holcomb, J. Trauma Acute Care Surg., 2006, 60, 655 CrossRef PubMed.
- D. R. Bhumkar, H. M. Joshi, M. Sastry and V. B. Pokharkar, J. Pharm. Res., 2007, 24, 1415 CrossRef CAS PubMed.
- S. YoungáChoi and S. YeongáLee, J. Mater. Chem., 2011, 21, 13853 RSC.
- R. Guo, L. Zhang, H. Qian, R. Li, X. Jiang and B. Liu, Langmuir, 2010, 26, 5428 CrossRef CAS PubMed.
- M. Mathew, S. Sureshkumar and N. Sandhyarani, Colloids Surf., B, 2012, 93, 143 CrossRef CAS PubMed.
- P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney and M. R. Boyd, J. Natl. Cancer Inst., 1990, 82, 1107 CrossRef CAS PubMed.
- S. C. Boca, M. Potara, F. Toderas, O. Stephan, P. L. Baldeck and S. Astilean, Mater. Sci. Eng., C, 2011, 31, 184 CrossRef CAS PubMed.
- G. Kroemer, W. S. El-Deiry, P. Golstein, M. E. Peter, D. Vaux, P. Vandenabeele, B. Zhivotovsky, M. V. Blagosklonny, W. Malorni, R. A. Knight, M. Piacentini, S. Nagata and G. Melino, Cell Death Differ., 2005, 12, 1463 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07551j |
| This journal is © The Royal Society of Chemistry 2014 |